A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis
- PMID: 14749422
- PMCID: PMC6729822
- DOI: 10.1523/JNEUROSCI.4488-03.2004
A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis
Abstract
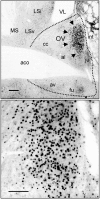
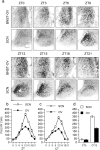
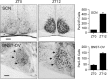
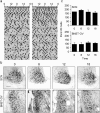
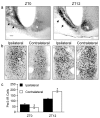
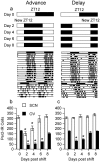
Circadian rhythms in mammals are regulated not only globally by the master clock in the suprachiasmatic nucleus (SCN), but also locally by widely distributed populations of clock cells in the brain and periphery that control tissue-specific rhythmic outputs. Here we show that the oval nucleus of the bed nucleus of the stria terminalis (BNST-OV) exhibits a robust circadian rhythm in expression of the Period2 (PER2) clock protein. PER2 expression is rhythmic in the BNST-OV in rats housed under a light/dark cycle or in constant darkness, in blind rats, and in mice, and is in perfect synchrony with the PER2 rhythm of the SCN. Constant light or bilateral SCN lesions abolish the rhythm of PER2 in the BNST-OV. Large abrupt shifts in the light schedule transiently uncouple the BNST-OV rhythm from that of the SCN. Re-entrainment of the PER2 rhythm is faster in the SCN than in the BNST-OV, and it is faster after a delay than an advance shift. Bilateral adrenalectomy blunts the PER2 rhythm in the BNST-OV. Thus, the BNST-OV contains circadian clock cells that normally oscillate in synchrony with the SCN, but these cells appear to require both input from the SCN and circulating glucocorticoids to maintain their circadian oscillation. Taken together with what is known about the functional organization of the connections of the BNST-OV with systems of the brain involved in stress and motivational processes, these findings place BNST-OV oscillators in a position to influence specific physiological and behavioral rhythms downstream from the SCN clock.
Figures


References
-
- Aguilar-Roblero R, Drucker-Colin R, Moore RY (1992) Behavioral and morphological studies of fetal neural transplants into SCN-lesioned rats. Chronobiol Int 9: 278–296. - PubMed
-
- Balsalobre A (2002) Clock genes in mammalian peripheral tissues. Cell Tissue Res 309: 193–199. - PubMed
-
- Balsalobre A, Marcacci L, Schibler U (2000a) Multiple signaling pathways elicit circadian gene expression in cultured rat-1 fibroblasts. Curr Biol 10: 1291–1294. - PubMed
-
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U (2000b) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
