The activation mechanism of alpha1 homomeric glycine receptors
- PMID: 14749434
- PMCID: PMC6729805
- DOI: 10.1523/JNEUROSCI.4420-03.2004
The activation mechanism of alpha1 homomeric glycine receptors
Abstract
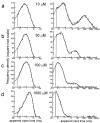
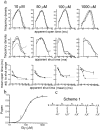
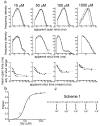
The glycine receptor mediates fast synaptic inhibition in the spinal cord and brainstem. Its activation mechanism is not known, despite the physiological importance of this receptor and the fact that it can serve as a prototype for other homopentameric channels. We analyzed single-channel recordings from rat recombinant alpha1 glycine receptors by fitting different mechanisms simultaneously to sets of sequences of openings at four glycine concentrations (10-1000 microm). The adequacy of the mechanism and the rate constants thus fitted was judged by examining how well these described the observed dwell-time distributions, open-shut correlation, and single-channel P(open) dose-response curve. We found that gating efficacy increased as more glycine molecules bind to the channel, but maximum efficacy was reached when only three (of five) potential binding sites are occupied. Successive binding steps are not identical, implying that binding sites can interact while the channel is shut. These interactions can be interpreted in the light of the topology of the binding sites within a homopentamer.
Figures

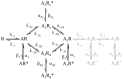

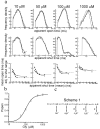
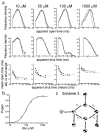
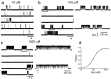
References
-
- Amin J, Weiss DS (1996) Insights into the activation mechanism of ρ1 GABA receptors obtained by coexpression of wild type and activation impaired subunits. Proc R Soc Lond B Biol Sci 263: 273–282. - PubMed
-
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van der Oost J, Smit AB, Sixma TK (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411: 269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
