Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain
- PMID: 14749444
- PMCID: PMC6729819
- DOI: 10.1523/JNEUROSCI.4792-03.2004
Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain
Abstract
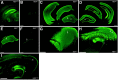
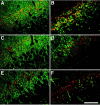
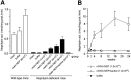
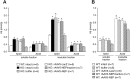
A local increase in amyloid-beta peptide (Abeta) is closely associated with synaptic dysfunction in the brain in Alzheimer's disease. Here, we report on the catabolic mechanism of Abeta at the presynaptic sites. Neprilysin, an Abeta-degrading enzyme, expressed by recombinant adeno-associated viral vector-mediated gene transfer, was axonally transported to presynaptic sites through afferent projections of neuronal circuits. This gene transfer abolished the increase in Abeta levels in the hippocampal formations of neprilysin-deficient mice and also reduced the increase in young mutant amyloid precursor protein transgenic mice. In the latter case, Abeta levels in the hippocampal formation contralateral to the vector-injected side were also significantly reduced as a result of transport of neprilysin from the ipsilateral side, and in both sides soluble Abeta was degraded more efficiently than insoluble Abeta. Furthermore, amyloid deposition in aged mutant amyloid precursor protein transgenic mice was remarkably decelerated. Thus, presynaptic neprilysin has been demonstrated to degrade Abeta efficiently and to retard development of amyloid pathology.
Figures
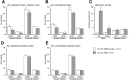
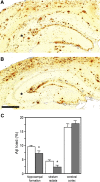
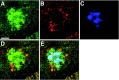
References
-
- Akiyama H, Kondo H, Ikeda K, Kato M, McGeer PL (2001) Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid β-protein (Aβ) deposition. Brain Res 902: 277–281. - PubMed
-
- Amaral DG, Witter MP (1995) Hippocampal formation. In: The rat nervous system (Paxinos G, ed), pp 443–493. San Diego: Academic.
-
- Barnes K, Turner AJ, Kenny AJ (1988) Electronmicroscopic immunocytochemistry of pig brain shows that endopeptidase-24.11 is localized in neuronal membranes. Neurosci Lett 94: 64–69. - PubMed
-
- Barnes K, Turner AJ, Kenny AJ (1992) Membrane localization of endopeptidase-24.11 and peptidyl dipeptidase A (angiotensin converting enzyme) in the pig brain: a study using subcellular fractionation and electron microscopic immunocytochemistry. J Neurochem 58: 2088–2096. - PubMed
-
- Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L (2002) Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid β peptides but not on plaque formation. J Neurosci 22: 10539–10548. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
