Analysis of Scc1-deficient cells defines a key metaphase role of vertebrate cohesin in linking sister kinetochores
- PMID: 14749720
- PMCID: PMC1298988
- DOI: 10.1038/sj.embor.7400077
Analysis of Scc1-deficient cells defines a key metaphase role of vertebrate cohesin in linking sister kinetochores
Abstract
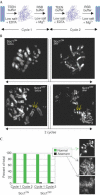
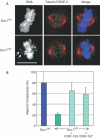
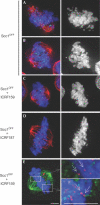
Cleavage of the cohesin subunit Scc1p/Mcd1p/Rad21 permits sister chromatid separation and is considered to trigger anaphase onset. It has also been suggested that the cohesin complex is essential for chromosome condensation and for assembling fully functional kinetochores. Here, we used vertebrate cells conditionally deficient in Scc1 to probe cohesin function in mitosis. Cells lacking cohesin arrest in prometaphase, with many chromosomes failing to align at a metaphase plate and high levels of the spindle assembly checkpoint protein, BubR1, at all kinetochores. We show that the structural integrity of chromosomes is normal in the absence of Scc1. Furthermore, specific inhibition of topoisomerase II, which is required for decatenation of replicated chromosomes, can bypass the cohesin requirement for metaphase chromosome alignment and spindle checkpoint silencing. Since the kinetochore effects of Scc1 deficiency can be compensated for by topoisomerase II inhibition, we conclude that Scc1 is not absolutely required for kinetochore assembly or function, and that its principal role in allowing the onset of anaphase is the establishment of sufficient inter-sister tension to allow biorientation.
Figures
References
-
- Andoh T, Ishida R (1998) Catalytic inhibitors of DNA topoisomerase II. Biochim Biophys Acta 1400: 155–171 - PubMed
-
- Bachant J, Alcasabas A, Blat Y, Kleckner N, Elledge SJ (2002) The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol Cell 9: 1169–1182 - PubMed
-
- Blat Y, Kleckner N (1999) Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98: 249–259 - PubMed
-
- Cole A (1967) Chromosome structure. Theor Biophys 1: 305–375
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

