The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore
- PMID: 14749836
- PMCID: PMC3049806
- DOI: 10.1038/nature02229
The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore
Abstract
A sudden increase in permeability of the inner mitochondrial membrane, the so-called mitochondrial permeability transition, is a common feature of apoptosis and is mediated by the mitochondrial permeability transition pore (mtPTP). It is thought that the mtPTP is a protein complex formed by the voltage-dependent anion channel, members of the pro- and anti-apoptotic BAX-BCL2 protein family, cyclophilin D, and the adenine nucleotide (ADP/ATP) translocators (ANTs). The latter exchange mitochondrial ATP for cytosolic ADP and have been implicated in cell death. To investigate the role of the ANTs in the mtPTP, we genetically inactivated the two isoforms of ANT in mouse liver and analysed mtPTP activation in isolated mitochondria and the induction of cell death in hepatocytes. Mitochondria lacking ANT could still be induced to undergo permeability transition, resulting in release of cytochrome c. However, more Ca2+ than usual was required to activate the mtPTP, and the pore could no longer be regulated by ANT ligands. Moreover, hepatocytes without ANT remained competent to respond to various initiators of cell death. Therefore, ANTs are non-essential structural components of the mtPTP, although they do contribute to its regulation.
Figures

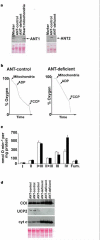
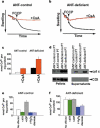
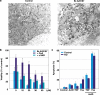
Comment in
-
Mitochondrial permeability: dual role for the ADP/ATP translocator?Nature. 2004 Aug 26;430(7003):1 p following 983. doi: 10.1038/nature02816. Nature. 2004. PMID: 15332302
References
-
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. - PubMed
-
- Marzo I, et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. - PubMed
-
- Levy SE, Chen Y-S, Graham BH, Wallace DC. Expression and sequence analysis of the mouse adenine nucleotide translocase 1 and 2 genes. Gene. 2000;254:57–66. - PubMed
-
- Ellison JW, Salido EC, Shapiro LJ. Genetic mapping of the adenine nucleotide translocase-2 gene (Ant2) to the mouse proximal X chromosome. Genomics. 1996;36:369–371. - PubMed
-
- Graham B, et al. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/skeletal muscle isoform of the adenine nucleotide translocator. Nature Genet. 1997;16:226–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

