Roles of Bifocal, Homer, and F-actin in anchoring Oskar to the posterior cortex of Drosophila oocytes
- PMID: 14752008
- PMCID: PMC324420
- DOI: 10.1101/gad.282604
Roles of Bifocal, Homer, and F-actin in anchoring Oskar to the posterior cortex of Drosophila oocytes
Abstract
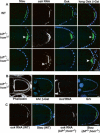
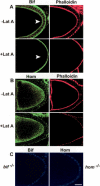
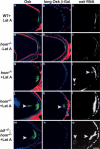
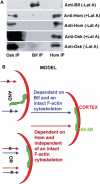
Transport, translation, and anchoring of osk mRNA and proteins are essential for posterior patterning of Drosophila embryos. Here we show that Homer and Bifocal act redundantly to promote posterior anchoring of the osk gene products. Disruption of actin microfilaments, which causes delocalization of Bifocal but not Homer from the oocyte cortex, severely disrupts anchoring of osk gene products only when Homer (not Bifocal) is absent. Our data suggest that two processes, one requiring Bifocal and an intact F-actin cytoskeleton and a second requiring Homer but independent of intact F-actin, may act redundantly to mediate posterior anchoring of the osk gene products.
Figures

References
-
- Brakeman P.R., Lanahan, A.A., O'Brien, R., Roche, K., Barnes, C.A., Huganir, R.L., and Worley, P.F. 1997. Homer: A protein that selectively binds metabotropic glutamate receptors. Nature 386: 284-288. - PubMed
-
- Broadus J. and Doe, C.Q. 1997. Extrinsic cues, intrinsic cues and microfilaments regulate asymmetric protein localization in Drosophila neuroblasts. Curr. Biol. 7: 827-835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
