Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes
- PMID: 14752101
- PMCID: PMC1817753
- DOI: 10.1074/jbc.M313837200
Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes
Abstract
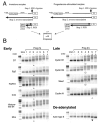
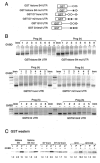
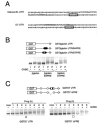
Meiotic cell cycle progression during vertebrate oocyte maturation requires the correct temporal translation of maternal mRNAs encoding key regulatory proteins. The mechanism by which specific mRNAs are temporally activated is unknown, although both cytoplasmic polyadenylation elements (CPE) within the 3'-untranslated region (3'-UTR) of mRNAs and the CPE-binding protein (CPEB) have been implicated. We report that in progesterone-stimulated Xenopus oocytes, the early cytoplasmic polyadenylation and translational activation of multiple maternal mRNAs occur in a CPE- and CPEB-independent manner. We demonstrate that polyadenylation response elements, originally identified in the 3'-UTR of the mRNA encoding the Mos proto-oncogene, direct CPE- and CPEB-independent polyadenylation of an early class of Xenopus maternal mRNAs. Our findings refute the hypothesis that CPE sequences alone account for the range of temporal inductions of maternal mRNAs observed during Xenopus oocyte maturation. Rather, our data indicate that the sequential action of distinct 3'-UTR-directed translational control mechanisms coordinates the complex temporal patterns and extent of protein synthesis during vertebrate meiotic cell cycle progression.
Figures




References
-
- Hochegger H, Klotzbucher A, Kirk J, Howell M, le Guellec K, Fletcher K, Duncan T, Sohail M, Hunt T. Development. 2001;128:3795–3807. - PubMed
-
- Sheets MD, Wu M, Wickens M. Nature. 1995;374:511–516. - PubMed
-
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. Curr Biol. 2000;10:430–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

