Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice
- PMID: 14755002
- PMCID: PMC1664879
- DOI: 10.1113/jphysiol.2003.059659
Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice
Erratum in
- J Physiol. 2004 May 1;556(Pt 3):1013
Abstract
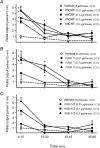
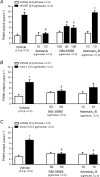
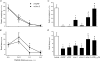
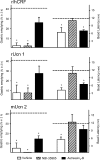
Recently characterized selective agonists and developed antagonists for the corticotropin releasing factor (CRF) receptors are new tools to investigate stress-related functional changes. The influence of mammalian CRF and related peptides injected intracerebroventricularly (i.c.v.) on gastric and colonic motility, and the CRF receptor subtypes involved and their role in colonic response to stress were studied in conscious mice. The CRF(1)/CRF(2) agonists rat urocortin 1 (rUcn 1) and rat/human CRF (r/h CRF), the preferential CRF(1) agonist ovine CRF (oCRF), and the CRF(2) agonist mouse (m) Ucn 2, injected i.c.v. inhibited gastric emptying and stimulated distal colonic motor function (bead transit and defecation) while oCRF(9-33)OH (devoid of CRF receptor affinity) showed neither effects. mUcn 2 injected peripherally had no colonic effect. The selective CRF(2) antagonist astressin(2)-B (i.c.v.), at a 20 : 1 antagonist: agonist ratio, blocked i.c.v. r/hCRF and rUcn 1 induced inhibition of gastric transit and reduced that of mUcn 2, while the CRF(1) antagonist NBI-35965 had no effect. By contrast, the colonic motor stimulation induced by i.c.v. r/hCRF and rUcn 1 and 1h restraint stress were antagonized only by NBI-35965 while stimulation induced by mUcn 2 was equally blocked by both antagonists. None of the CRF antagonists injected i.c.v. alone influenced gut transit. These data establish in mice that brain CRF(1) receptors mediate the stimulation of colonic transit induced by central CRF, urocortins (1 and 2) and restraint stress, while CRF(2) receptors mediate the inhibitory actions of these peptides on gastric transit.
Figures

Similar articles
-
Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2.J Pharmacol Exp Ther. 2002 May;301(2):611-7. doi: 10.1124/jpet.301.2.611. J Pharmacol Exp Ther. 2002. PMID: 11961064
-
Human urocortin II, a new CRF-related peptide, displays selective CRF(2)-mediated action on gastric transit in rats.Am J Physiol Gastrointest Liver Physiol. 2002 Jan;282(1):G34-40. doi: 10.1152/ajpgi.00283.2001. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 11751155
-
Intracisternal urocortin inhibits vagally stimulated gastric motility in rats: role of CRF(2).Br J Pharmacol. 2002 May;136(2):237-47. doi: 10.1038/sj.bjp.0704713. Br J Pharmacol. 2002. PMID: 12010772 Free PMC article.
-
Urocortins and the regulation of gastrointestinal motor function and visceral pain.Peptides. 2004 Oct;25(10):1733-44. doi: 10.1016/j.peptides.2004.05.025. Peptides. 2004. PMID: 15476940 Review.
-
Corticotropin-releasing factor and the brain-gut motor response to stress.Can J Gastroenterol. 1999 Mar;13 Suppl A:18A-25A. doi: 10.1155/1999/375916. Can J Gastroenterol. 1999. PMID: 10202204 Review.
Cited by
-
Brain-Gut Interactions in IBS.Front Pharmacol. 2012 Jul 5;3:127. doi: 10.3389/fphar.2012.00127. eCollection 2012. Front Pharmacol. 2012. PMID: 22783191 Free PMC article.
-
Peripheral α2-β1 adrenergic interactions mediate the ghrelin response to brain urocortin 1 in rats.Psychoneuroendocrinology. 2014 Dec;50:300-10. doi: 10.1016/j.psyneuen.2014.09.003. Epub 2014 Sep 16. Psychoneuroendocrinology. 2014. PMID: 25265283 Free PMC article.
-
Exploring the Role of Urocortin in Osteoporosis.Cureus. 2023 May 13;15(5):e38978. doi: 10.7759/cureus.38978. eCollection 2023 May. Cureus. 2023. PMID: 37313093 Free PMC article. Review.
-
Water-avoidance stress enhances gastric contractions in freely moving conscious rats: role of peripheral CRF receptors.J Gastroenterol. 2014 May;49(5):799-805. doi: 10.1007/s00535-013-0828-8. Epub 2013 May 5. J Gastroenterol. 2014. PMID: 23645119
-
Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system.J Comp Neurol. 2006 Jan 1;494(1):63-74. doi: 10.1002/cne.20781. J Comp Neurol. 2006. PMID: 16304680 Free PMC article.
References
-
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer's disease. Nature. 1995;378:284–287. - PubMed
-
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

