Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice
- PMID: 14755333
- PMCID: PMC324538
- DOI: 10.1172/JCI19448
Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice
Abstract
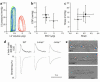
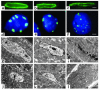
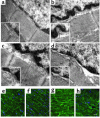
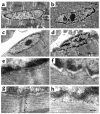
Laminopathies are a group of disorders caused by mutations in the LMNA gene that encodes the nuclear lamina proteins, lamin A and lamin C; their pathophysiological basis is unknown. We report that lamin A/C-deficient (Lmna(-/-)) mice develop rapidly progressive dilated cardiomyopathy (DCM) characterized by left ventricular (LV) dilation and reduced systolic contraction. Isolated Lmna(-/-) myocytes show reduced shortening with normal baseline and peak amplitude of Ca(2+) transients. Lmna(-/-) LV myocyte nuclei have marked alterations of shape and size with central displacement and fragmentation of heterochromatin; these changes are present but less severe in left atrial nuclei. Electron microscopy of Lmna(-/-) cardiomyocytes shows disorganization and detachment of desmin filaments from the nuclear surface with progressive disruption of the cytoskeletal desmin network. Alterations in nuclear architecture are associated with defective nuclear function evidenced by decreased SREBP1 import, reduced PPARgamma expression, and a lack of hypertrophic gene activation. These findings suggest a model in which the primary pathophysiological mechanism in Lmna(-/-) mice is defective force transmission resulting from disruption of lamin interactions with the muscle-specific desmin network and loss of cytoskeletal tension. Despite severe DCM, defects in nuclear function prevent Lmna(-/-) cardiomyocytes from developing compensatory hypertrophy and accelerate disease progression.
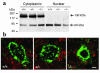
Figures


Comment in
-
How do mutations in lamins A and C cause disease?J Clin Invest. 2004 Feb;113(3):349-51. doi: 10.1172/JCI20832. J Clin Invest. 2004. PMID: 14755330 Free PMC article. Review.
References
-
- Hozak P, Sasseville AM, Raymond Y, Cook PR. Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in human cells. J. Cell Sci. 1995;108:635–644. - PubMed
-
- Barboro P, et al. Unravelling the organization of the internal nuclear matrix: RNA-dependent anchoring of NuMA to a lamin scaffold. Exp. Cell Res. 2002;279:202–218. - PubMed
-
- Manilal S, et al. Distribution of emerin and lamins in the heart and implications for Emery-Dreifuss muscular dystrophy. Hum. Mol. Genet. 1999;8:353–359. - PubMed
-
- Stuurman N, Heins S, Aebi U. Nuclear lamins: their structure, assembly and interactions. J. Struct. Biol. 1998;122:42–66. - PubMed
-
- Burke B, Stewart CL. Life at the edge: the nuclear envelope and human disease. Nat. Rev. Mol. Cell Biol. 2002;3:575–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

