Site and mechanism of leptin action in a rodent form of congenital lipodystrophy
- PMID: 14755338
- PMCID: PMC324539
- DOI: 10.1172/JCI19511
Site and mechanism of leptin action in a rodent form of congenital lipodystrophy
Abstract
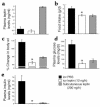
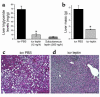
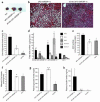
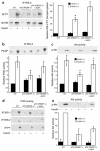
Lipodystrophy is characterized by the complete or partial absence of adipose tissue, insulin resistance, hepatic steatosis, and leptin deficiency. Here, we show that low-dose central leptin corrects the insulin resistance and fatty liver of lipodystrophic aP2-nSREBP-1c mice, while the same dose given peripherally does not. Central leptin also repressed stearoyl-CoA desaturase-1 (SCD-1) RNA and enzymatic activity, which were increased in livers of lipodystrophic mice. aP2-nSREBP-1c mice homozygous for an SCD-1 deletion had markedly reduced hepatic steatosis, increased saturated fatty acids, decreased acetyl-CoA carboxylase activity, and decreased malonyl-CoA levels in the liver. Despite the reduction in hepatic steatosis, these mice remained diabetic. A leptin dose-response curve showed that subcutaneous leptin improved hyperglycemia and hyperinsulinemia in aP2-nSREBP-1c mice at doses that did not substantially alter hepatic steatosis or hepatic SCD enzymatic activity. Leptin treatment at this dose improved insulin-stimulated insulin receptor and insulin receptor substrate 2 (IRS-2) phosphorylation, IRS-2-associated PI3K activity, and Akt activity in liver. Together, these data suggest that CNS-mediated repression of SCD-1 contributes to leptin's antisteatotic actions. Intracerebroventricular leptin improves glucose homeostasis by improving insulin signal transduction in liver, but in this case the effect appears to be independent of SCD-1.
Figures



References
-
- Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. - PubMed
-
- Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. - PubMed
-
- Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492. - PubMed
-
- Ogawa Y, et al. Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes. 1999;48:1822–1829. - PubMed
-
- Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

