Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay
- PMID: 14757700
- PMCID: PMC1574235
- DOI: 10.1038/sj.bjp.0705652
Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay
Abstract
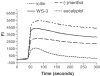
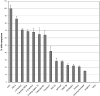
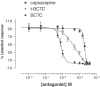
1. TRPM8 (CMR1) is a Ca(2+)-permeable channel, which can be activated by low temperatures, menthol, eucalyptol and icilin. It belongs to the transient receptor potential (TRP) family, and therefore is related to vanilloid receptor type-1 (VR1, TRPV1). We tested whether substances which are structurally related to menthol, or which produce a cooling sensation, could activate TRPM8, and compared the responses of TRPM8 and VR1 to these ligands. 2. The effects of 70 odorants and menthol-related substances on recombinant mouse TRPM8 (mTRPM8), expressed in HEK293 cells, were examined using a FLIPR assay. In all, 10 substances (linalool, geraniol, hydroxycitronellal, WS-3, WS-23, FrescolatMGA, FrescolatML, PMD38, CoolactP and Cooling Agent 10) were found to be agonists. 3. The EC(50) values of the agonists defined their relative potencies: icilin (0.2+/-0.1 microM)>FrescolatML (3.3+/-1.5 microM) > WS-3 (3.7+/-1.7 microM) >(-)menthol (4.1+/-1.3 microM) >frescolatMAG (4.8+/-1.1 microM) > cooling agent 10 (6+/-2.2 microM) >(+)menthol (14.4+/-1.3 microM) > PMD38 (31+/-1.1 microM) > WS-23 (44+/-7.3 microM) > Coolact P (66+/-20 microM) > geraniol (5.9+/-1.6 mM) > linalool (6.7+/-2.0 mM) > eucalyptol (7.7+/-2.0 mM) > hydroxycitronellal (19.6+/-2.2 mM). 4. Known VR1 antagonists (BCTC, thio-BCTC and capsazepine) were also able to block the response of TRPM8 to menthol (IC(50): 0.8+/-1.0, 3.5+/-1.1 and 18+/-1.1 microM, respectively). 5. The Ca(2+) response of hVR1-transfected HEK293 cells to the endogenous VR1 agonist N-arachidonoyl-dopamine was potentiated by low pH. In contrast, menthol- and icilin-activated TRPM8 currents were suppressed by low pH. 6. In conclusion, in the present study, we identified 10 new agonists and three antagonists of TRPM8. We found that, in contrast to VR1, TRPM8 is inhibited rather than potentiated by protons.
Figures

References
-
- BISOGNO T., MELCK D., BOBROV M.YU., GRETSKAYA N.M., BEZUGLOV V.V., DE PETROCELLIS L., DI MARZO V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351:817–824. - PMC - PubMed
-
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
-
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- DAVIES J.S., HARDING L.M., BARANOWSKI A.P. A novel treatment of postherpetic neuralgia using peppermint oil. Clin. J. Pain. 2002;8:200–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

