Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death
- PMID: 14757745
- PMCID: PMC2211792
- DOI: 10.1084/jem.20032092
Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death
Abstract
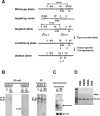
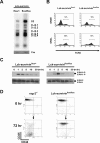
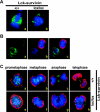
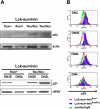
Because survivin-null embryos die at an early embryonic stage, the role of survivin in thymocyte development is unknown. We have investigated the role by deleting the survivin gene only in the T lineage and show here that loss of survivin blocks the transition from CD4- CD8- double negative (DN) thymocytes to CD4+ CD8+ double positive cells. Although the pre-T cell receptor signaling pathway is intact in survivin-deficient thymocytes, the cells cannot respond to its signals. In response to proliferative stimuli, cycling survivin-deficient DN cells exhibit cell cycle arrest, a spindle formation defect, and increased cell death. Strikingly, loss of survivin activates the tumor suppressor p53. However, the developmental defects caused by survivin deficiency cannot be rescued by p53 inactivation or introduction of Bcl-2. These lines of evidence indicate that developing thymocytes depend on the cytoprotective function of survivin and that this function is tightly coupled to cell proliferation but independent of p53 and Bcl-2. Thus, survivin plays a critical role in early thymocyte development.
Figures



Similar articles
-
p53 prevents maturation of T cell development to the immature CD4-CD8+ stage in Bcl11b-/- mice.Biochem Biophys Res Commun. 2005 Mar 11;328(2):545-9. doi: 10.1016/j.bbrc.2005.01.013. Biochem Biophys Res Commun. 2005. PMID: 15694382
-
p53-dependent and p53-independent pathways for radiation-induced immature thymocyte differentiation.Oncogene. 2004 Mar 11;23(10):1922-9. doi: 10.1038/sj.onc.1207320. Oncogene. 2004. PMID: 14755249
-
Expression of CD226 antagonizes apoptotic cell death in murine thymocytes.J Immunol. 2009 May 1;182(9):5453-60. doi: 10.4049/jimmunol.0803090. J Immunol. 2009. PMID: 19380793
-
The role of p53 in neuronal cell death.Cell Death Differ. 2000 Oct;7(10):868-79. doi: 10.1038/sj.cdd.4400741. Cell Death Differ. 2000. PMID: 11279532 Review.
-
Survivin apoptosis: an interloper between cell death and cell proliferation in cancer.Lab Invest. 1999 Nov;79(11):1327-33. Lab Invest. 1999. PMID: 10576203 Review. No abstract available.
Cited by
-
The detergent-soluble cytoplasmic pool of survivin suppresses anoikis and its expression is associated with metastatic disease of human colon cancer.PLoS One. 2013;8(2):e55710. doi: 10.1371/journal.pone.0055710. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405201 Free PMC article.
-
The tumor gene survivin is highly expressed in adult renal tubular cells: implications for a pathophysiological role in the kidney.Am J Pathol. 2007 Nov;171(5):1483-98. doi: 10.2353/ajpath.2007.070132. Am J Pathol. 2007. PMID: 17982126 Free PMC article.
-
p53-induced apoptosis occurs in the absence of p14(ARF) in malignant pleural mesothelioma.Neoplasia. 2006 Jul;8(7):551-9. doi: 10.1593/neo.06148. Neoplasia. 2006. PMID: 16867217 Free PMC article.
-
Perinatal survivin is essential for the establishment of pancreatic beta cell mass in mice.Diabetologia. 2009 Oct;52(10):2130-41. doi: 10.1007/s00125-009-1469-6. Epub 2009 Jul 31. Diabetologia. 2009. PMID: 19644667
-
Survivin safeguards chromosome numbers and protects from aneuploidy independently from p53.Mol Cancer. 2014 May 9;13:107. doi: 10.1186/1476-4598-13-107. Mol Cancer. 2014. PMID: 24886358 Free PMC article.
References
-
- Godfrey, D.I., J. Kennedy, T. Suda, and A. Zlotnik. 1993. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 150:4244–4252. - PubMed
-
- Dudley, E.C., H.T. Petrie, L.M. Shah, M.J. Owen, and A.C. Hayday. 1994. T cell receptor beta chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1:83–93. - PubMed
-
- Levelt, C.N., and K. Eichmann. 1995. Receptors and signals in early thymic selection. Immunity. 3:667–672. - PubMed
-
- von Boehmer, H., I. Aifantis, J. Feinberg, O. Lechner, C. Saint-Ruf, U. Walter, J. Buer, and O. Azogui. 1999. Pleiotropic changes controlled by the pre-T-cell receptor. Curr. Opin. Immunol. 11:135–142. - PubMed
-
- Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I.L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 89:1033–1041. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

