Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death
- PMID: 14757751
- PMCID: PMC2172235
- DOI: 10.1083/jcb.200307132
Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death
Abstract
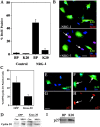
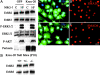
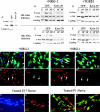
The transcription factor Krox-20 controls Schwann cell myelination. Schwann cells in Krox-20 null mice fail to myelinate, and unlike myelinating Schwann cells, continue to proliferate and are susceptible to death. We find that enforced Krox-20 expression in Schwann cells cell-autonomously inactivates the proliferative response of Schwann cells to the major axonal mitogen beta-neuregulin-1 and the death response to TGFbeta or serum deprivation. Even in 3T3 fibroblasts, Krox-20 not only blocks proliferation and death but also activates the myelin genes periaxin and protein zero, showing properties in common with master regulatory genes in other cell types. Significantly, a major function of Krox-20 is to suppress the c-Jun NH2-terminal protein kinase (JNK)-c-Jun pathway, activation of which is required for both proliferation and death. Thus, Krox-20 can coordinately control suppression of mitogenic and death responses. Krox-20 also up-regulates the scaffold protein JNK-interacting protein 1 (JIP-1). We propose this as a possible component of the mechanism by which Krox-20 regulates JNK activity during Schwann cell development.
Copyright The Rockefeller University Press
Figures
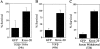






References
-
- Awatramani, R., S. Shumas, J. Kamholz, and S.S. Scherer. 2002. TGFbeta1 modulates the phenotype Schwann cells at the transcriptional level. Mol. Cell. Neurosci. 19:307–319. - PubMed
-
- Archelos, J.J., K. Roggenbuck, J. Schneider-Schaulies, C. Linington, K.V. Toyka, and H.P. Hartung. 1993. Production and characterization of monoclonal antibodies to the extracellular domain of P0. J. Neurosci. Res. 35:46–53. - PubMed
-
- Bonny, C., A. Oberson, M. Steinmann, D.F. Schorderet, P. Nicod, and G. Waeber. 2000. IB1 reduces cytokine-induced apoptosis of insulin-secreting cells. J. Biol. Chem. 275:16466–16472. - PubMed
-
- Brockes, J.P., K.L. Fields, and M.C. Raff. 1979. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 165:105–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

