Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly
- PMID: 14757753
- PMCID: PMC2172230
- DOI: 10.1083/jcb.200306032
Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly
Abstract
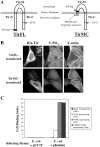
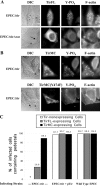
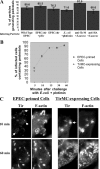
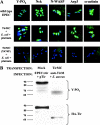
Enteropathogenic Escherichia coli (EPEC) translocates effector proteins into mammalian cells to promote reorganization of the cytoskeleton into filamentous actin pedestals. One effector, Tir, is a transmembrane receptor for the bacterial surface adhesin intimin, and intimin binding by the extracellular domain of Tir is required for actin assembly. The cytoplasmic NH2 terminus of Tir interacts with focal adhesion proteins, and its tyrosine-phosphorylated COOH terminus binds Nck, a host adaptor protein critical for pedestal formation. To define the minimal requirements for EPEC-mediated actin assembly, Tir derivatives were expressed in mammalian cells in the absence of all other EPEC components. Replacement of the NH2 terminus of Tir with a viral membrane-targeting sequence promoted efficient surface expression of a COOH-terminal Tir fragment. Artificial clustering of this fusion protein revealed that the COOH terminus of Tir, by itself, is sufficient to initiate a complete signaling cascade leading to pedestal formation. Consistent with this finding, clustering of Nck by a 12-residue Tir phosphopeptide triggered actin tail formation in Xenopus egg extracts.
Copyright The Rockefeller University Press
Figures



Similar articles
-
Nck adaptors, besides promoting N-WASP mediated actin-nucleation activity at pedestals, influence the cellular levels of enteropathogenic Escherichia coli Tir effector.Cell Adh Migr. 2014;8(4):404-17. doi: 10.4161/19336918.2014.969993. Cell Adh Migr. 2014. PMID: 25482634 Free PMC article.
-
EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly.Dev Cell. 2004 Aug;7(2):217-28. doi: 10.1016/j.devcel.2004.07.004. Dev Cell. 2004. PMID: 15296718
-
Enterohaemorrhagic and enteropathogenic Escherichia coli Tir proteins trigger a common Nck-independent actin assembly pathway.Cell Microbiol. 2007 Sep;9(9):2242-53. doi: 10.1111/j.1462-5822.2007.00954.x. Epub 2007 May 23. Cell Microbiol. 2007. PMID: 17521329
-
Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157:H7.Curr Opin Microbiol. 2003 Feb;6(1):82-90. doi: 10.1016/s1369-5274(03)00005-5. Curr Opin Microbiol. 2003. PMID: 12615225 Review.
-
Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal.Cell Microbiol. 2008 Mar;10(3):549-56. doi: 10.1111/j.1462-5822.2007.01103.x. Epub 2007 Dec 4. Cell Microbiol. 2008. PMID: 18053003 Review.
Cited by
-
Cytoskeletal mechanisms regulating attaching/effacing bacteria interactions with host cells: It takes a village to build the pedestal.Bioessays. 2024 Nov;46(11):e2400160. doi: 10.1002/bies.202400160. Epub 2024 Sep 20. Bioessays. 2024. PMID: 39301984 Review.
-
Enteropathogenic Escherichia coli O125:H6 triggers attaching and effacing lesions on human intestinal biopsy specimens independently of Nck and TccP/TccP2.Infect Immun. 2008 Jan;76(1):361-8. doi: 10.1128/IAI.01199-07. Epub 2007 Nov 5. Infect Immun. 2008. PMID: 17984209 Free PMC article.
-
Attaching-and-Effacing Pathogens Exploit Junction Regulatory Activities of N-WASP and SNX9 to Disrupt the Intestinal Barrier.Cell Mol Gastroenterol Hepatol. 2017 Dec 15;5(3):273-288. doi: 10.1016/j.jcmgh.2017.11.015. eCollection 2018 Mar. Cell Mol Gastroenterol Hepatol. 2017. PMID: 29675452 Free PMC article.
-
Nck adaptors at a glance.J Cell Sci. 2021 Sep 15;134(18):jcs258965. doi: 10.1242/jcs.258965. Epub 2021 Sep 24. J Cell Sci. 2021. PMID: 34558601 Free PMC article.
-
A reciprocal interdependence between Nck and PI(4,5)P(2) promotes localized N-WASp-mediated actin polymerization in living cells.Mol Cell. 2009 Nov 13;36(3):525-35. doi: 10.1016/j.molcel.2009.10.025. Mol Cell. 2009. PMID: 19917259 Free PMC article.
References
-
- Abe, A., M. de Grado, R.A. Pfuetzner, C. Sanchez-SanMartin, R. DeVinney, J.L. Puente, N.C.J. Strynadka, and B.B. Finlay. 1999. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol. Microbiol. 33:1162–1175. - PubMed
-
- Bladt, F., E. Aippersbach, S. Gelkop, G.A. Strasser, P. Nash, A. Tafuri, F.B. Gertler, and T. Pawson. 2003. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol. Cell. Biol. 23:4586–4597. - PMC - PubMed
-
- Buday, L., L. Wunderlich, and P. Tamas. 2002. The Nck family of adapter proteins. Regulators of actin cytoskeleton. Cell. Signal. 14:723–731. - PubMed
-
- Campellone, K.G., A. Giese, D.J. Tipper, and J.M. Leong. 2002. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol. Microbiol. 43:1227–1241. - PubMed
-
- Cantarelli, V.V., A. Takahashi, I. Yanagihara, Y. Akeda, K. Imura, T. Kodama, G. Kono, Y. Sato, and T. Honda. 2001. Talin, a host cell protein, interacts directly with the translocated intimin receptor, Tir, of enteropathogenic Escherichia coli, and is essential for pedestal formation. Cell. Microbiol. 3:745–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

