The metabolic world of Escherichia coli is not small
- PMID: 14757824
- PMCID: PMC341771
- DOI: 10.1073/pnas.0306458101
The metabolic world of Escherichia coli is not small
Abstract
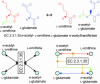
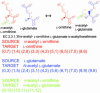
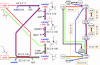
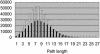
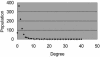
To elucidate the organizational and evolutionary principles of the metabolism of living organisms, recent studies have addressed the graph-theoretic analysis of large biochemical networks responsible for the synthesis and degradation of cellular building blocks [Jeong, H., Tombor, B., Albert, R., Oltvai, Z. N. & Barabási, A. L. (2000) Nature 407, 651-654; Wagner, A. & Fell, D. A. (2001) Proc. R. Soc. London Ser. B 268, 1803-1810; and Ma, H.-W. & Zeng, A.-P. (2003) Bioinformatics 19, 270-277]. In such studies, the global properties of the network are computed by considering enzymatic reactions as links between metabolites. However, the pathways computed in this manner do not conserve their structural moieties and therefore do not correspond to biochemical pathways on the traditional metabolic map. In this work, we reassessed earlier results by digitizing carbon atomic traces in metabolic reactions annotated for Escherichia coli. Our analysis revealed that the average path length of its metabolism is much longer than previously thought and that the metabolic world of this organism is not small in terms of biosynthesis and degradation.
Figures
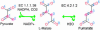
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

