Ca2+/calmodulin transfers the membrane-proximal lipid-binding domain of the v-SNARE synaptobrevin from cis to trans bilayers
- PMID: 14757830
- PMCID: PMC341777
- DOI: 10.1073/pnas.0303274101
Ca2+/calmodulin transfers the membrane-proximal lipid-binding domain of the v-SNARE synaptobrevin from cis to trans bilayers
Abstract
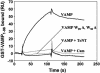
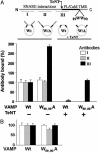
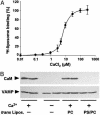
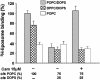
Soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) protein interactions at the synaptic vesicle/plasma membrane interface play an essential role in neurotransmitter release. The membrane-proximal region (amino acids 77-90) of the v-SNARE vesicle-associated membrane protein 2 (VAMP 2, synaptobrevin) binds acidic phospholipids or Ca(2+)/calmodulin in a mutually exclusive manner, processes that are required for Ca(2+)-dependent exocytosis. To address the mechanisms involved, we asked whether this region of VAMP can interact with cis (outer vesicle leaflet) and/or trans (inner plasma membrane leaflet) lipids. To evaluate cis lipid binding, recombinant VAMP was reconstituted into liposomes and accessibility to site-directed antibodies was probed by surface plasmon resonance. Data indicated that the membrane-proximal domain of VAMP dips into the cis lipid bilayer, sequestering epitopes between the tetanus toxin cleavage site and the membrane anchor. These epitopes were unmasked by VAMP double mutation W89A, W90A, which abolishes lipid interactions. To evaluate trans lipid binding, VAMP was reconstituted in cis liposomes, which were then immobilized on beads. The ability of VAMP to capture protein-free (3)H-labeled trans liposomes was then measured. When cis lipid interactions were eliminated by omitting negatively charged lipids, trans lipid binding to VAMP was revealed. In contrast, when cis and trans liposomes both contained acidic headgroups (i.e., approximating physiological conditions), cis lipid interactions totally occluded trans lipid binding. In these conditions Ca(2+)/calmodulin displaced cis inhibition, transferring the lipid-binding domain of VAMP from the cis to the trans bilayer. Our results suggest that calmodulin acts as a unidirectional Ca(2+)-activated shuttle that docks the juxtamembrane portion of the v-SNARE in the target membrane to prepare fusion.
Figures

References
-
- Sollner, T., Whiteheart, S. W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P. & Rothman, J. E. (1993) Nature 362, 318-324. - PubMed
-
- McNew, J. A., Parlati, F., Fukuda, R., Johnston, R. J., Paz, K., Paumet, F., Sollner, T. H. & Rothman, J. E. (2000) Nature 407, 153-159. - PubMed
-
- Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T. H. & Rothman, J. E. (1998) Cell 92, 759-772. - PubMed
-
- Jahn, R. & Südhof, T. C. (1999) Annu. Rev. Biochem. 68, 863-911. - PubMed
-
- Mayer, A. (1999) Curr. Opin. Cell Biol. 11, 447-452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

