Chloride ligation in inorganic manganese model compounds relevant to photosystem II studied using X-ray absorption spectroscopy
- PMID: 14758524
- PMCID: PMC3965209
- DOI: 10.1007/s00775-003-0520-1
Chloride ligation in inorganic manganese model compounds relevant to photosystem II studied using X-ray absorption spectroscopy
Abstract
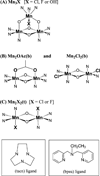
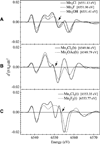
Chloride ions are essential for proper function of the photosynthetic oxygen-evolving complex (OEC) of Photosystem II (PS II). Although proposed to be directly ligated to the Mn cluster of the OEC, the specific structural and mechanistic roles of chloride remain unresolved. This study utilizes X-ray absorption spectroscopy (XAS) to characterize the Mn-Cl interaction in inorganic compounds that contain structural motifs similar to those proposed for the OEC. Three sets of model compounds are examined; they possess core structures Mn(IV)(3)O(4)X (X=Cl, F, or OH) that contain a di-micro-oxo and two mono-micro-oxo bridges or Mn(IV)(2)O(2)X (X=Cl, F, OH, OAc) that contain a di-micro-oxo bridge. Each set of compounds is examined for changes in the XAS spectra that are attributable to the replacement of a terminal OH or F ligand, or bridging OAc ligand, by a terminal Cl ligand. The X-ray absorption near edge structure (XANES) shows changes in the spectra on replacement of OH, OAc, or F by Cl ligands that are indicative of the overall charge of the metal atom and are consistent with the electronegativity of the ligand atom. Fourier transforms (FTs) of the extended X-ray absorption fine structure (EXAFS) spectra reveal a feature that is present only in compounds where chloride is directly ligated to Mn. These FT features were simulated using various calculated Mn-X interactions (X=O, N, Cl, F), and the best fits were found when a Mn-Cl interaction at a 2.2-2.3 A bond distance was included. There are very few high-valent Mn halide complexes that have been synthesized, and it is important to make such a comparative study of the XANES and EXAFS spectra because they have the potential for providing information about the possible presence or absence of halide ligation to the Mn cluster in PS II.
Figures




Similar articles
-
Mn K-edge XANES and Kbeta XES studies of two Mn-oxo binuclear complexes: investigation of three different oxidation states relevant to the oxygen-evolving complex of photosystem II.J Am Chem Soc. 2001 Jul 25;123(29):7031-9. doi: 10.1021/ja004306h. J Am Chem Soc. 2001. PMID: 11459481 Free PMC article.
-
Structural consequences of ammonia binding to the manganese center of the photosynthetic oxygen-evolving complex: an X-ray absorption spectroscopy study of isotropic and oriented photosystem II particles.Biochemistry. 1995 Apr 18;34(15):5274-87. doi: 10.1021/bi00015a043. Biochemistry. 1995. PMID: 7711049
-
Comparison of the Manganese Cluster in Oxygen-Evolving Photosystem II with Distorted Cubane Manganese Compounds through X-ray Absorption Spectroscopy.Inorg Chem. 1999 Dec 27;38(26):5988-5998. doi: 10.1021/ic991003j. Inorg Chem. 1999. PMID: 11671305 Free PMC article.
-
An evaluation of structural models for the photosynthetic water-oxidizing complex derived from spectroscopic and X-ray diffraction signatures.J Biol Inorg Chem. 2002 Jan;7(1-2):2-22. doi: 10.1007/s00775-001-0305-3. Epub 2001 Nov 8. J Biol Inorg Chem. 2002. PMID: 11862536 Review.
-
S1-state Mn4Ca complex of Photosystem II exists in equilibrium between the two most-stable isomeric substates: XRD and EXAFS evidence.J Photochem Photobiol B. 2011 Jul-Aug;104(1-2):100-10. doi: 10.1016/j.jphotobiol.2011.03.002. Epub 2011 Mar 13. J Photochem Photobiol B. 2011. PMID: 21592813 Review.
Cited by
-
Tribute to Kenneth Sauer (1931-2022): a mentor, a role-model, and an inspiration to all in the field of photosynthesis.Photosynth Res. 2024 Dec;162(2-3):103-138. doi: 10.1007/s11120-024-01119-0. Epub 2024 Nov 13. Photosynth Res. 2024. PMID: 39535662 Free PMC article.
-
Absence of Mn-centered oxidation in the S(2) --> S(3) transition: implications for the mechanism of photosynthetic water oxidation.J Am Chem Soc. 2001 Aug 15;123(32):7804-20. doi: 10.1021/ja004307+. J Am Chem Soc. 2001. PMID: 11493054 Free PMC article.
-
Functional Models for the Oxygen-Evolving Complex of Photosystem II.Coord Chem Rev. 2008 Feb 1;252(3-4):444-455. doi: 10.1016/j.ccr.2007.06.002. Coord Chem Rev. 2008. PMID: 21037800 Free PMC article.
-
Structure and orientation of the Mn4Ca cluster in plant photosystem II membranes studied by polarized range-extended x-ray absorption spectroscopy.J Biol Chem. 2007 Mar 9;282(10):7198-208. doi: 10.1074/jbc.M610505200. Epub 2006 Dec 26. J Biol Chem. 2007. PMID: 17190828 Free PMC article.
-
Structure of the Mn4-Ca cluster as derived from X-ray diffraction.Photosynth Res. 2007 Jun;92(3):389-405. doi: 10.1007/s11120-007-9173-1. Epub 2007 May 11. Photosynth Res. 2007. PMID: 17492491 Review.
References
-
- Kok B, Forbush B, McGloin M. Photochem Photobiol. 1970;11:457–475. - PubMed
-
- Zouni A, Witt H-T, Kern J, Fromme P, Krauß N, Saenger W, Orth P. Nature. 2001;409:739–743. - PubMed
-
- Yachandra VK, DeRose VJ, Latimer MJ, Mukerji I, Sauer K, Klein MP. Science. 1993;260:675–679. - PubMed
-
- Yachandra VK, Sauer K, Klein MP. Chem Rev. 1996;96:2927–2950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

