gp41: HIV's shy protein
- PMID: 14760422
- PMCID: PMC7095992
- DOI: 10.1038/nm0204-133
gp41: HIV's shy protein
Abstract
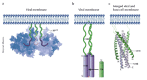
The first X-ray crystal structures of gp41, the protein that mediates fusion of HIV-1 to target cells, were solved in the mid-1990s. The structures provide a foundation for understanding viral entry and the mechanism of action of compounds that block fusion. The first fusion inhibitor has recently entered the clinic, and the hope is that more potent and broadly active compounds, based on molecular design, will follow.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

