The Bacillus subtilis extracytoplasmic-function sigmaX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides
- PMID: 14762009
- PMCID: PMC344218
- DOI: 10.1128/JB.186.4.1136-1146.2004
The Bacillus subtilis extracytoplasmic-function sigmaX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides
Abstract
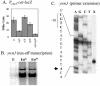
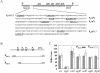
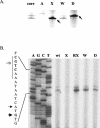
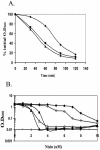
Bacillus subtilis contains seven extracytoplasmic-function sigma factors that activate partially overlapping regulons. We here identify four additional members of the sigma(X) regulon, pbpX (penicillin-binding protein), ywnJ, the dlt operon (D-alanylation of teichoic acids), and the pss ybfM psd operon (phosphatidylethanolamine biosynthesis). Modification of teichoic acids by esterification with D-alanine and incorporation of phosphatidylethanolamine into the cell membrane have a common consequence: in both cases positively charged amino groups are introduced into the cell envelope. The resulting reduction in the net negative charge of the cell envelope has been previously implicated as a resistance mechanism specific for cationic antimicrobial peptides. Consistent with this notion, we find that both sigX and dltA mutants are more sensitive to nisin than wild-type cells. We conclude that activation of the sigma(X) regulon serves to alter cell surface properties to provide protection against antimicrobial peptides.
Figures




References
-
- Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol Microbiol. 43:1-14. - PubMed
-
- Akbar, S., and C. W. Price. 1996. Isolation and characterization of csbB, a gene controlled by Bacillus subtilis general stress transcription factor σB. Gene 177:123-128. - PubMed
-
- Brutsche, S., and V. Braun. 1997. SigX of Bacillus subtilis replaces the ECF sigma factor FecI of Escherichia coli and is inhibited by RsiX. Mol. Gen. Genet. 256:416-425. - PubMed
-
- Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

