Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers
- PMID: 14764894
- PMCID: PMC357048
- DOI: 10.1073/pnas.0307301101
Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers
Abstract
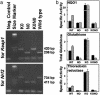
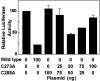
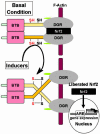
Induction of a family of phase 2 genes encoding for proteins that protect against the damage of electrophiles and reactive oxygen intermediates is potentially a major strategy for reducing the risk of cancer and chronic degenerative diseases. Many phase 2 genes are regulated by upstream antioxidant response elements (ARE) that are targets of the leucine zipper transcription factor Nrf2. Under basal conditions, Nrf2 resides mainly in the cytoplasm bound to its cysteine-rich, Kelch domain-containing partner Keap1, which is itself anchored to the actin cytoskeleton and represses Nrf2 activity. Inducers disrupt the Keap1-Nrf2 complex by modifying two (C273 and C288) of the 25 cysteine residues of Keap1. The critical role of C273 and C288 was established by (i) their high reactivity when purified recombinant Keap1 was treated with dexamethasone mesylate and the dexamethasone-modified tryptic peptides were analyzed by mass spectrometry, and (ii) transfection of keap1 and nrf2 gene-deficient mouse embryonic fibroblasts with constructs expressing cysteine to alanine mutants of Keap1, and measurement of the ability of cotransfected Nrf2 to repress an ARE-luciferase reporter. Reaction of Keap1 with inducers results in formation of intermolecular disulfide bridges, probably between C273 of one Keap1 molecule and C288 of a second. Evidence for formation of such dimers was obtained by 2D PAGE of extracts of cells treated with inducers, and by the demonstration that whereas C273A and C288A mutants of Keap1 alone could not repress Nrf2 activation of the ARE-luciferase reporter, an equal mixture of these mutant constructs restored repressor activity.
Figures

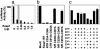


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

