A troponin switch that regulates muscle contraction by stretch instead of calcium
- PMID: 14765112
- PMCID: PMC381005
- DOI: 10.1038/sj.emboj.7600097
A troponin switch that regulates muscle contraction by stretch instead of calcium
Abstract
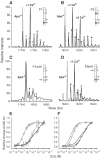
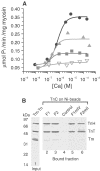
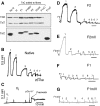
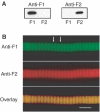
The flight muscles of many insects have a form of regulation enabling them to contract at high frequencies. The muscles are activated by periodic stretches at low Ca2+ levels. The same muscles also give isometric contractions in response to higher Ca2+. We show that the two activities are controlled by different isoforms of TnC (F1 and F2) within single myofibrils. F1 binds one Ca2+ with high affinity in the C-terminal domain and F2 binds one Ca2+ in the C-terminal domain and one exchangeable Ca2+ in the N-terminal domain. We have characterised the isoforms and determined their effect on the development of stretch-activated and Ca2+-activated tension by replacing endogenous TnC in Lethocerus flight muscle fibres with recombinant isoforms. Fibres with F1 gave stretch-activated tension and minimal isometric tension; those with F2 gave Ca2+-dependent isometric tension and minimal stretch-activated tension. Regulation by a TnC responding to stretch rather than Ca2+ is unprecedented and has resulted in the ability of insect flight muscle to perform oscillatory work at low Ca2+ concentrations, a property to which a large number of flying insects owe their evolutionary success.
Figures



References
-
- Allhouse LD, Potter JD, Ashley CC (1999) A novel method of extraction of TnC from skeletal muscle myofibrils. Pflügers Arch 437: 695–701 - PubMed
-
- Bers DM, Patton CW, Nuccitelli R (1994) A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol 40: 3–29 - PubMed
-
- Bullard B, Bell J, Craig R, Leonard K (1985) Arthrin: a new actin-like protein in insect flight muscle. J Mol Biol 182: 443–454 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

