The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly
- PMID: 14765128
- PMCID: PMC380990
- DOI: 10.1038/sj.emboj.7600066
The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly
Abstract
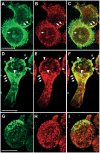
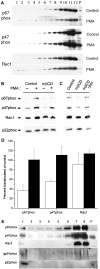
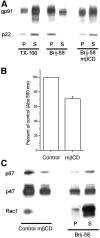
The superoxide-producing phagocyte NADPH oxidase consists of a membrane-bound flavocytochrome b558 complex, and cytosolic factors p47phox, p67phox and the small GTPase Rac, which translocate to the membrane to assemble the active complex following cell activation. We here show that insolubility of NADPH oxidase subunits in nonionic detergents TX-100, Brij-58, and Brij-98 is a consequence of inclusion into cholesterol-enriched membrane microdomains (lipid rafts). Thus, flavocytochrome b558, in a cholesterol-dependent manner, segregated to the bouyant low-density detergent-resistant membrane (DRM) fraction, and the cytosolic NADPH oxidase factors associated dynamically with low-density DRM. Further, superoxide production following cholesterol depletion was severely compromised in intact cells or in a cell-free reconstituted system, correlating with a reduced translocation of cytosolic phox subunits to the membrane. In analogy with the widely accepted role of lipid rafts as signaling platforms, our data indicate that cholesterol-enriched microdomains act to recruit and/or organize the cytosolic NADPH oxidase factors in the assembly of the active NADPH oxidase.
Figures





Similar articles
-
Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts.J Biol Chem. 2005 Nov 4;280(44):37021-32. doi: 10.1074/jbc.M506594200. Epub 2005 Aug 22. J Biol Chem. 2005. PMID: 16115878
-
Targeting of Rac1 to the phagocyte membrane is sufficient for the induction of NADPH oxidase assembly.J Biol Chem. 2000 Dec 22;275(51):40073-81. doi: 10.1074/jbc.M006013200. J Biol Chem. 2000. PMID: 11007780
-
Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task.Small GTPases. 2014;5:e27952. doi: 10.4161/sgtp.27952. Epub 2014 Mar 5. Small GTPases. 2014. PMID: 24598074 Free PMC article. Review.
-
p21rac does not participate in the early interaction between p47-phox and cytochrome b558 that leads to phagocyte NADPH oxidase activation in vitro.Biochemistry. 1994 Mar 8;33(9):2490-5. doi: 10.1021/bi00175a018. Biochemistry. 1994. PMID: 8117710
-
Assembly of the phagocyte NADPH oxidase.Histochem Cell Biol. 2004 Oct;122(4):277-91. doi: 10.1007/s00418-004-0679-8. Epub 2004 Aug 4. Histochem Cell Biol. 2004. PMID: 15293055 Review.
Cited by
-
Proteomic characterization of phagosomal membrane microdomains during phagolysosome biogenesis and evolution.Mol Cell Proteomics. 2012 Nov;11(11):1365-77. doi: 10.1074/mcp.M112.021048. Epub 2012 Aug 20. Mol Cell Proteomics. 2012. PMID: 22915823 Free PMC article.
-
Localization and functional characterization of the pathogenesis-related proteins Rbe1p and Rbt4p in Candida albicans.PLoS One. 2018 Aug 6;13(8):e0201932. doi: 10.1371/journal.pone.0201932. eCollection 2018. PLoS One. 2018. PMID: 30080909 Free PMC article.
-
Lovastatin inhibits human B lymphoma cell proliferation by reducing intracellular ROS and TRPC6 expression.Biochim Biophys Acta. 2014 May;1843(5):894-901. doi: 10.1016/j.bbamcr.2014.02.002. Epub 2014 Feb 8. Biochim Biophys Acta. 2014. PMID: 24518247 Free PMC article.
-
Reactive oxygen species, Ki-Ras, and mitochondrial superoxide dismutase cooperate in nerve growth factor-induced differentiation of PC12 cells.J Biol Chem. 2010 Jul 30;285(31):24141-53. doi: 10.1074/jbc.M109.098525. Epub 2010 May 21. J Biol Chem. 2010. PMID: 20495008 Free PMC article.
-
Hydrogen peroxide-induced translocation of glycolipid-anchored (c)AMP-hydrolases to lipid droplets mediates inhibition of lipolysis in rat adipocytes.Br J Pharmacol. 2008 Jun;154(4):901-13. doi: 10.1038/bjp.2008.146. Epub 2008 May 5. Br J Pharmacol. 2008. PMID: 18454169 Free PMC article.
References
-
- Ago T, Nunoi H, Ito T, Sumimoto H (1999) Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox). Triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J Biol Chem 274: 33644–33653 - PubMed
-
- Allen LA, DeLeo FR, Gallois A, Toyoshima S, Suzuki K, Nauseef WM (1999) Transient association of the nicotinamide adenine dinucleotide phosphate oxidase subunits p47phox and p67phox with phagosomes in neutrophils from patients with X-linked chronic granulomatous disease. Blood 93: 3521–3530 - PubMed
-
- Bodin S, Giuriato S, Ragab J, Humbel BM, Viala C, Vieu C, Chap H, Payrastre B (2001) Production of phosphatidylinositol 3,4,5-trisphosphate and phosphatidic acid in platelet rafts: evidence for a critical role of cholesterol-enriched domains in human platelet activation. Biochemistry 40: 15290–15299 - PubMed
-
- Bokoch GM, Bohl BP, Chuang TH (1994) Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem 269: 31674–31679 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

