Degradation pathway and generation of monohydroxamic acids from the trihydroxamate siderophore deferrioxamine B
- PMID: 14766561
- PMCID: PMC348822
- DOI: 10.1128/AEM.70.2.831-836.2004
Degradation pathway and generation of monohydroxamic acids from the trihydroxamate siderophore deferrioxamine B
Abstract
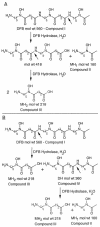
Siderophores are avid ferric ion-chelating molecules that sequester the metal for microbes. Microbes elicit siderophores in numerous and different environments, but the means by which these molecules reenter the carbon and nitrogen cycles is poorly understood. The metabolism of the trihydroxamic acid siderophore deferrioxamine B by a Mesorhizobium loti isolated from soil was investigated. Specifically, the pathway by which the compound is cleaved into its constituent monohydroxamates was examined. High-performance liquid chromatography and mass-spectroscopy analyses demonstrated that M. loti enzyme preparations degraded deferrioxamine B, yielding a mass-to-charge (m/z) 361 dihydroxamic acid intermediate and an m/z 219 monohydroxamate. The dihydroxamic acid was further degraded to yield a second molecule of the m/z 219 monohydroxamate as well as an m/z 161 monohydroxamate. These studies indicate that the dissimilation of deferrioxamine B by M. loti proceeds by a specific, achiral degradation and likely represents the reversal by which hydroxamate siderophores are thought to be synthesized.
Figures

References
-
- Beare, P. A., R. J. For, L. W. Martin, and I. L. Lamont. 2003. Siderophore-mediated cell signaling in Pseudomonas aeruginosa: divergent pathways regulate virulence factor production and siderophore receptor synthesis. Mol. Microbiol. 47:195-207. - PubMed
-
- Bellaire, B. H., P. H. Elzer, S. Hagius, J. Walker, C. L. Baldwin, and R. M. Roop. 2003. Genetic organization and iron-responsive regulation of the Brucella abortus 2,3-dihydroxybenzoic acid biosynthesis operon, a cluster of genes required for wild-type virulence in pregnant cattle. Infect. Immun. 71:1794-1803. - PMC - PubMed
-
- Castignetti, D., and A. S. Siddiqui. 1990. The catabolism and heterotrophic nitrification of the siderophore deferrioxamine B. BioMetals 3:197-203. - PubMed
-
- Castignetti, D., A. S. Siddiqui, and K. W. Olsen. 1988. A spectrophotometric assay for the degradation of the siderophore deferrioxamine B. J. Biochem. Biophys. Methods 17:119-126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

