Viral preprotoxin signal sequence allows efficient secretion of green fluorescent protein by Candida glabrata, Pichia pastoris, Saccharomyces cerevisiae, and Schizosaccharomyces pombe
- PMID: 14766577
- PMCID: PMC348933
- DOI: 10.1128/AEM.70.2.961-966.2004
Viral preprotoxin signal sequence allows efficient secretion of green fluorescent protein by Candida glabrata, Pichia pastoris, Saccharomyces cerevisiae, and Schizosaccharomyces pombe
Abstract
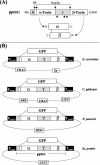
Besides its importance as model organism in eukaryotic cell biology, yeast species have also developed into an attractive host for the expression, processing, and secretion of recombinant proteins. Here we investigated foreign protein secretion in four distantly related yeasts (Candida glabrata, Pichia pastoris, Saccharomyces cerevisiae, and Schizosaccharomyces pombe) by using green fluorescent protein (GFP) as a reporter and a viral secretion signal sequence derived from the K28 preprotoxin (pptox), the precursor of the yeast K28 virus toxin. In vivo expression of GFP fused to the N-terminal pptox leader sequence and/or expression of the entire pptox gene was driven either from constitutive (PGK1 and TPI1) or from inducible and/or repressible (GAL1, AOX1, and NMT1) yeast promoters. In each case, GFP entered the secretory pathway of the corresponding host cell; confocal fluorescence microscopy as well as sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western analysis of cell-free culture supernatants confirmed that GFP was efficiently secreted into the culture medium. In addition to the results seen with GFP, the full-length viral pptox was correctly processed in all four yeast genera, leading to the secretion of a biologically active virus toxin. Taken together, our data indicate that the viral K28 pptox signal sequence has the potential for being used as a unique tool in recombinant protein production to ensure efficient protein secretion in yeast.
Figures


References
-
- Bader, O., M. Schaller, S. Klein, J. Kukula, K. Haack, F. Muhlschlegel, H. C. Korting, W. Schafer, and B. Hube. 2001. The KEX2 gene of Candida glabrata is required for cell surface integrity. Mol. Microbiol. 41:1431-1444. - PubMed
-
- Boehm, T., S. Pirie-Shephard, L. B. Trinh, J. Shiloach, and J. Folkman. 1999. Disruption of the KEX1 gene in Pichia pastoris allows expression of full-length murine and human endostatin. Yeast 15:563-572. - PubMed
-
- Braspenning, J., W. Meschede, A. Marchini, M. Muller, L. Gissmann, and M. Tommasino. 1998. Secretion of heterologous proteins from Schizosaccharomyces pombe using the homologous leader sequence of pho1+ acid phosphatase. Biochem. Biophys. Res. Commun. 245:166-171. - PubMed
-
- Buckholz, R. G., and M. A. Gleeson. 1991. Yeast systems for the commercial production of heterologous proteins. Biotechnology 9:1067-1072. - PubMed
-
- Campbell, T. N., and F. Y. M. Choy. 2002. Expression of two green fluorescent protein variants in citrate-buffered media in Pichia pastoris. Anal. Biochem. 311:193-195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

