Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes
- PMID: 14766612
- PMCID: PMC348862
- DOI: 10.1128/AEM.70.2.1234-1237.2004
Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes
Abstract
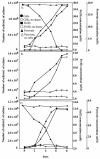
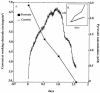
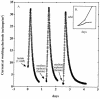
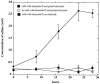
Desulfobulbus propionicus was able to grow with Fe(III), the humic acids analog anthraquinone-2,6-disulfonate (AQDS), or a graphite electrode as an electron acceptor. These results provide an explanation for the enrichment of Desulfobulbaceae species on the surface of electrodes harvesting electricity from anaerobic marine sediments and further expand the diversity of microorganisms known to have the ability to use both sulfate and Fe(III) as an electron acceptor.
Figures
References
-
- Aller, R. C., and P. D. Rude. 1988. Complete oxidation of solid phase sulfides by manganese and bacteria in anoxic marine sediments. Geochim. Cosmochim. Acta 52:751-765.
-
- Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. - PubMed
-
- Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. - PubMed
-
- Dannenberg, S., M. Kroder, W. Dilling, and H. Cypionka. 1992. Oxidation of H2, organic compounds and inorganic sulfur compounds coupled to reduction of O2 or nitrate by sulfate-reducing bacteria. Arch. Microbiol. 158:93-99.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

