Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries
- PMID: 14766935
- PMCID: PMC1664993
- DOI: 10.1113/jphysiol.2003.059568
Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries
Abstract
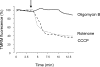
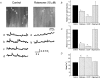
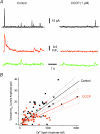
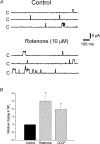
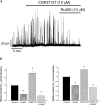
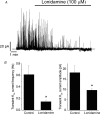
Mitochondria sequester and release calcium (Ca(2+)) and regulate intracellular Ca(2+) concentration ([Ca(2+)](i)) in eukaryotic cells. However, the regulation of different Ca(2+) signalling modalities by mitochondria in smooth muscle cells is poorly understood. Here, we investigated the regulation of Ca(2+) sparks, Ca(2+) waves and global [Ca(2+)](i) by mitochondria in cerebral artery smooth muscle cells. CCCP (a protonophore; 1 microm) and rotenone (an electron transport chain complex I inhibitor; 10 microm) depolarized mitochondria, reduced Ca(2+) spark and wave frequency, and elevated global [Ca(2+)](i) in smooth muscle cells of intact arteries. In voltage-clamped (-40 mV) cells, mitochondrial depolarization elevated global [Ca(2+)](i), reduced Ca(2+) spark amplitude, spatial spread and the effective coupling of sparks to large-conductance Ca(2+)-activated potassium (K(Ca)) channels, and decreased transient K(Ca) current frequency and amplitude. Inhibition of Ca(2+) sparks and transient K(Ca) currents by mitochondrial depolarization could not be explained by a decrease in intracellular ATP or a reduction in sarcoplasmic reticulum Ca(2+) load, and occurred in the presence of diltiazem, a voltage-dependent Ca(2+) channel blocker. Ru360 (10 microm), a mitochondrial Ca(2+) uptake blocker, and lonidamine (100 microm), a permeability transition pore (PTP) opener, inhibited transient K(Ca) currents similarly to mitochondrial depolarization. In contrast, CGP37157 (10 microm), a mitochondrial Na(+)-Ca(2+) exchange blocker, activated these events. The PTP blockers bongkrekic acid and cyclosporin A both reduced inhibition of transient K(Ca) currents by mitochondrial depolarization. These results indicate that mitochondrial depolarization leads to a voltage-independent elevation in global [Ca(2+)](i) and Ca(2+) spark and transient K(Ca) current inhibition. Data also suggest that mitochondrial depolarization inhibits Ca(2+) sparks and transient K(Ca) currents via PTP opening and a decrease in intramitochondrial [Ca(2+)].
Figures




References
-
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. - PubMed
-
- Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–H1289. - PubMed
-
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

