Astrocyte-mediated activation of neuronal kainate receptors
- PMID: 14766987
- PMCID: PMC365762
- DOI: 10.1073/pnas.0306731101
Astrocyte-mediated activation of neuronal kainate receptors
Abstract
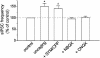
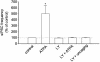
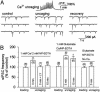
Exogenous kainate receptor agonists have been shown to modulate inhibitory synaptic transmission in the hippocampus, but the pathways involved in physiological activation of the receptors remain largely unknown. Accumulating evidence indicates that astrocytes can release glutamate in a Ca(2+)-dependent manner and signal to neighboring neurons. We tested the hypothesis that astrocyte-derived glutamate activates kainate receptors on hippocampal interneurons. We report here that elevation of intracellular Ca(2+) in astrocytes, induced by uncaging Ca(2+), o-nitrophenyl-EGTA, increased action potential-driven spontaneous inhibitory postsynaptic currents in nearby interneurons in rat hippocampal slices. This effect was blocked by alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate glutamate receptor antagonists, but not by selective AMPA receptor or N-methyl-d-aspartate receptor antagonists. This pharmacological profile indicates that kainate receptors were activated during Ca(2+) elevation in astrocytes. Kainate receptors containing the GluR5 subunit seemed to mediate the observed effect because a selective GluR5-containing kainate receptor antagonist blocked the changes in sIPSCs induced by Ca(2+) uncaging, and bath application of a selective GluR5-containing receptor agonist robustly potentiated sIPSCs. When tetrodotoxin was included to block action potentials, Ca(2+) uncaging induced a small decrease in the frequency of miniature inhibitory postsynaptic currents, which was not affected by AMPA/kainate receptor antagonists. Our data suggest that an astrocyte-derived, nonsynaptic source of glutamate represents a signaling pathway that can activate neuronal kainate receptors. By modulating the activity of interneurons, astrocytes may play a critical role in circuit function of hippocampus.
Figures


Comment in
-
When astrocytes signal, kainate receptors respond.Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):2649-50. doi: 10.1073/pnas.0400474101. Epub 2004 Feb 23. Proc Natl Acad Sci U S A. 2004. PMID: 14981250 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

