Rapid, endoplasmic reticulum-independent diffusion of the mitotic Golgi haze
- PMID: 14767069
- PMCID: PMC379280
- DOI: 10.1091/mbc.e03-07-0459
Rapid, endoplasmic reticulum-independent diffusion of the mitotic Golgi haze
Abstract
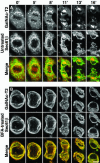
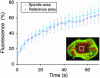
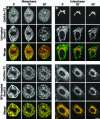
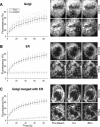
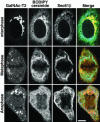
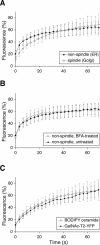
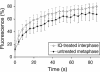
Early in mitosis, the mammalian Golgi apparatus disassembles, and fluorescence microscopy reveals Golgi clusters and an extensive, nonresolvable haze that either represents scattered vesicles or a merged endoplasmic reticulum (ER)-Golgi compartment. To help decide between these alternatives, we have carried out a combined microscopic and pharmacological analysis, by using a BS-C-1 cell line stably coexpressing ER and Golgi markers. Video fluorescence microscopy showed that these two organelles were morphologically distinguishable at all stages of mitosis, and photobleaching experiments showed that diffusion of the Golgi marker was unaffected by the presence of the ER. Fragmentation of the ER by using filipin III completely blocked diffusion of the ER marker but had no effect on the Golgi marker, unless it was first relocated to the ER by using brefeldin A. The Golgi haze was also studied using BODIPY ceramide. Its diffusion was slower in mitotic Golgi than in mitotic ER, but similar to that of a Golgi enzyme marker in the mitotic Golgi haze or in Golgi vesicles generated by ilimaquinone. Together, these results support the idea that the Golgi and the ER remain separate during mitosis and strongly suggest that Golgi markers move by vesicle diffusion, as opposed to lateral diffusion in continuous membranes.
Figures
References
-
- Bevis, B.J., Hammond, A.T., Reinke, C.A., and Glick, B.S. (2002). De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat. Cell Biol. 4, 750-756. - PubMed
-
- Birky, C.W. (1983). The partitioning of cytoplasmic organelles at cell division. Int. Rev. Cytol. 15, 49-89. - PubMed
-
- Farmaki, T., Ponnambalam, S., Prescott, A.R., Clausen, H., Tang, B.L., Hong, W., and Lucocq, J.M. (1999). Forward and retrograde trafficking in mitotic animal cells. ER-Golgi transport arrest restricts protein export from the E.R. into COPII-coated structures. J. Cell Sci. 112, 589-600. - PubMed
-
- Glick, B.S., and Malhotra, V. (1998). The curious status of the Golgi apparatus. Cell 95, 883-889. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

