Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97-11
- PMID: 14769136
- PMCID: PMC1871974
- DOI: 10.1215/s1152851703000218
Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97-11
Abstract
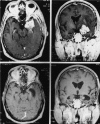
The primary objective of this study was to determine the proportion of patients exhibiting a radiographic response in a cohort of patients with recurrent malignant glioma who were treated with irinotecan. Secondary objectives were to determine progression-free survival, overall survival, and toxicity. The trial was terminated after the first 18 patients were enrolled in this multicenter, 2-stage, phase 2 study. Twelve patients received concurrent enzyme-inducing antiepileptic drugs, and 6 did not. Each cycle consisted of a 90-min i.v. infusion of irinotecan every week for 4 consecutive weeks, followed by 2 weeks off. One patient had a complete response, 5 patients had stable disease, 5 patients had radiographic progression, 6 patients were removed from the study because of toxicity, and 1 patient refused further therapy and was removed from the study. The response rate in this study was 6% (1/18), and 28% (5/18) of these patients progressed while receiving irinotecan. Dose-limiting toxicities consisted of diarrhea in 5 patients, neutropenia in 1 patient, infection in 1 patient, and respiratory failure in 1 patient. Irinotecan had minimal efficacy in this cohort of 18 patients with recurrent malignant glioma. Toxicity was significant but similar to that reported in other patient populations.
Figures

References
-
- Armand JP, Extra YM, Catimel G, Abigerges D, Marty M, Clavel M. Rationale for the dosage and schedule of CPT-11 (irinotecan) selected for phase II studies, as determined by European phase I studies. Ann Oncol. 1996;7:837–842. - PubMed
-
- Bleiberg H, Cvitkovic E. Characterisation and clinical management of CPT-11 (irinotecan)-induced adverse events: The European perspective. Eur J Cancer. 1996;32A:S18–S23. - PubMed
-
- CBTRUS. Central Brain Tumor Registry of the United States (2002). Statistical Report: Primary Brain Tumors in the United States, 1995 –1999. Chicago: Central Brain Tumor Registry of the United States (available at http://www.cbtrus.org/2002/2002report.pdf).
-
- Cloughesy T, Filka E, Friedman H, Kabbinavar F, Kuhn J, Selch M, Miller L. A phase I (intrapatient dose escalation) open-label study of irinotecan (CPT-11) in patients with recurrent or progressive malignant glioma. 19. 2000;Proc Am Soc Clin Oncol:161a. (abstract)
-
- Coggins CA, Elion GB, Houghton PJ, Hare CB, Keir S, Colvin OM, Bigner DD, Friedman HS. Enhancement of irinotecan (CPT-11) activity against central nervous system tumor xenografts by alkylating agents. Cancer Chemother Pharmacol. 1998;41:485–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

