Specificity of charge-carrying residues in the voltage sensor of potassium channels
- PMID: 14769847
- PMCID: PMC2217452
- DOI: 10.1085/jgp.200308993
Specificity of charge-carrying residues in the voltage sensor of potassium channels
Abstract
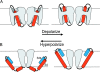
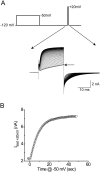
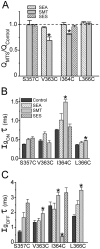
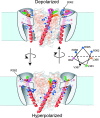
Positively charged voltage sensors of sodium and potassium channels are driven outward through the membrane's electric field upon depolarization. This movement is coupled to channel opening. A recent model based on studies of the KvAP channel proposes that the positively charged voltage sensor, christened the "voltage-sensor paddle", is a peripheral domain that shuttles its charged cargo through membrane lipid like a hydrophobic cation. We tested this idea by attaching charged adducts to cysteines introduced into the putative voltage-sensor paddle of Shaker potassium channels and measuring fractional changes in the total gating charge from gating currents. The only residues capable of translocating attached charges through the membrane-electric field are those that serve this function in the native channel. This remarkable specificity indicates that charge movement involves highly specialized interactions between the voltage sensor and other regions of the protein, a mechanism inconsistent with the paddle model.
Figures






References
-
- Aggarwal, S.K., and R. MacKinnon. 1996. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 16:1169–1177. - PubMed
-
- Baker, O.S., H.P. Larsson, L.M. Mannuzzu, and E.Y. Isacoff. 1998. Three transmembrane conformations and sequence-dependent displacement of the S4 domain in Shaker K+ channel gating. Neuron. 20:1283–1294. - PubMed
-
- Bezanilla, F. 2000. The voltage sensor in voltage dependent ion channels. Physiol. Rev. 80:555–592. - PubMed
-
- Blaustein, R.O., P.A. Cole, C. Williams, and C. Miller. 2000. Tethered blockers as molecular ‘tape measures’ for a voltage-gated K+ channel. Nat. Struct. Biol. 7:309–311. - PubMed

