In Candida albicans, the Nim1 kinases Gin4 and Hsl1 negatively regulate pseudohypha formation and Gin4 also controls septin organization
- PMID: 14769857
- PMCID: PMC2171991
- DOI: 10.1083/jcb.200307176
In Candida albicans, the Nim1 kinases Gin4 and Hsl1 negatively regulate pseudohypha formation and Gin4 also controls septin organization
Abstract
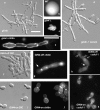
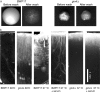
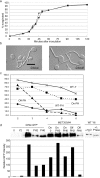
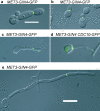
In the development of hyphal germ tubes of Candida albicans, a band of septin forms at the base of the germ tube (basal septin band). Later, a septin ring forms, which organizes the first septum within the germ tube (septin ring). We have investigated the role of the Nim1 kinases, Gin4 and Hsl1, in the formation of these septin structures. We show that during germ tube formation, Gin4 is required for the organization of the septin ring but not the basal septin band. Hsl1 is not required for the formation of either septin rings or basal bands. Unexpectedly, we found that both gin4Delta and hsl1Delta mutants form pseudohyphae constitutively, in a fashion that in the case of gin4Delta, is partly independent of Swe1. Gin4-depleted pseudohyphae are unable to form hyphae when challenged with serum, but this can be overcome by ectopic expression of Gin4 from the MET3 promoter. Thus, Gin4 may regulate the developmental switch from pseudohyphae to hyphae.
Copyright The Rockefeller University Press
Figures






References
-
- Berman, J., and P.E. Sudbery. 2002. Candida albicans: A molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

