Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis
- PMID: 14769925
- PMCID: PMC357043
- DOI: 10.1073/pnas.0400148101
Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis
Abstract
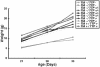
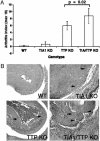
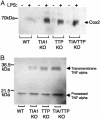
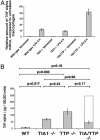
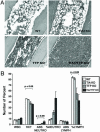
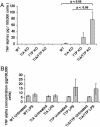
TIA-1 and TTP are AU-rich element-binding proteins that prevent the pathological overexpression of tumor necrosis factor alpha (TNF-alpha). TIA-1 inhibits the translation of TNF-alpha transcripts, whereas TTP promotes the degradation of TNF-alpha transcripts. Here we show that TIA-1 and TTP function as arthritis suppressor genes: TIA-1(-/-) mice develop mild arthritis, TTP(-/-) mice develop severe arthritis, and TIA-1(-/-)TTP(-/-) mice develop very severe arthritis. Peritoneal macrophages derived from all three genotypes overexpress cyclooxygenase 2 and TNF-alpha. Surprisingly, lipopolysaccharide-activated TIA-1(-/-)TTP(-/-) macrophages secrete less TNF-alpha protein than either TIA-1(-/-) or TTP(-/-) macrophages. In these mice, arthritogenic cytokine may be produced by neutrophils that accumulate in the bone marrow and peripheral blood. Our results suggest that TIA-1 and TTP are genetic modifiers of inflammatory arthritis that can alter the spectrum of cells that produce arthritogenic cytokines.
Figures
References
-
- Cope, A., Aderka, D., Doherty, M., Englemann, H., Gibbons, D., Jones, A., Brennan, F., Maini, R., Wallach, D. & Feldmann, M. (1992) Arthritis Rheum. 35, 1160–1169. - PubMed
-
- Feldmann, M. & Maini, R. N. (2002) Joint Bone Spine 69, 12–18. - PubMed
-
- Dreyfuss, G., Kim, V. N. & Kataoka, N. (2002) Nat. Rev. Mol. Cell Biol. 3, 195–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

