Modulation of mammalian life span by the short isoform of p53
- PMID: 14871929
- PMCID: PMC338283
- DOI: 10.1101/gad.1162404
Modulation of mammalian life span by the short isoform of p53
Abstract
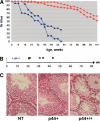
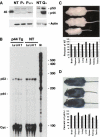
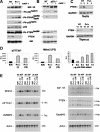
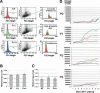
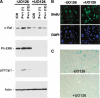
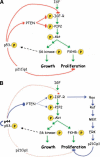
Overexpression of the short isoform of p53 (p44) has unexpectedly uncovered a role for p53 in the regulation of size and life span in the mouse. Hyperactivation of the insulin-like growth factor (IGF) signaling axis by p44 sets in motion a kinase cascade that clamps potentially unimpeded growth through p21Cip1. This suggests that pathways of gene activity known to regulate longevity in lower organisms are linked in mammals via p53 to mechanisms for controlling cell proliferation. Thus, appropriate expression of the short and long p53 isoforms might maintain a balance between tumor suppression and tissue regeneration, a major requisite for long mammalian life span.
Figures



References
-
- Bluher M., Kahn, B.B., and Kahn, C.R. 2003. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572-574. - PubMed
-
- Clancy D.J., Gems, D., Harshman, L.G., Oldham, S., Stocker, H., Hafen, E., Leevers, S.J., and Partridge, L. 2001. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292: 104-106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
