The Ca2+/calcineurin-regulated cup gene family in Dictyostelium discoideum and its possible involvement in development
- PMID: 14871937
- PMCID: PMC329516
- DOI: 10.1128/EC.3.1.61-71.2004
The Ca2+/calcineurin-regulated cup gene family in Dictyostelium discoideum and its possible involvement in development
Abstract
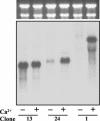
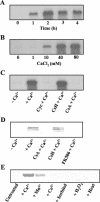
Changes in free intracellular Ca2+ are thought to regulate several major processes during Dictyostelium development, including cell aggregation and cell type-specific gene expression, but the mechanisms involved are unclear. To learn more about Ca2+ signaling and Ca2+ homeostasis in this organism, we used suppression subtractive hybridization to identify genes up-regulated by high extracellular Ca2+. Unexpectedly, many of the genes identified belong to a novel gene family (termed cup) with seven members. In vegetative cells, the cup genes were up-regulated by high Ca2+ but not by other ions or by heat, oxidative, or osmotic stress. cup induction by Ca2+ was blocked completely by inhibitors of calcineurin and protein synthesis. In developing cells, cup expression was high during aggregation and late development but low during the slug stage. This pattern correlates closely with reported levels of free intracellular Ca2+ during development. The cup gene products are highly homologous, acidic proteins possessing putative ricin domains. BLAST searches failed to reveal homologs in other organisms, but Western analyses suggested that Cup-like proteins might exist in certain other cellular slime mold species. Localization experiments indicated that Cup proteins are primarily cytoplasmic but become cell membrane-associated during Ca2+ stress and cell aggregation. When cup expression was down-regulated by antisense RNA, the cells failed to aggregate. However, this developmental block was overcome by partially up-regulating cup expression. Together, these results suggest that the Cup proteins in Dictyostelium might play an important role in stabilizing and/or regulating the cell membrane during Ca2+ stress and/or certain stages of development.
Figures






References
-
- Abe, T., and Y. Maeda. 1989. The prestalk/prespore differentiation and polarized cell movement in Dictyostelium discoideum slugs. A possible involvement of the intracellular Ca2+ concentration. Protoplasma 151:175-178.
-
- Aichem, A., and R. Mutzel. 2001. Unconventional mRNA processing in the expression of two calcineurin B isoforms in Dictyostelium. J. Mol. Biol. 308:873-882. - PubMed
-
- Baskar, R., P. Chhabra, P. Mascarenhas, and V. Nanjundiah. 2000. A cell type-specific effect of calcium on pattern formation and differentiation in Dictyostelium discoideum. Ind. J. Dev. Biol. 44:491-498. - PubMed
-
- Blumberg, D., J. F. Comer, and E. M. Walton. 1989. Ca++ antagonists distinguish different requirements for cAMP-mediated gene expression in the cellular slime mold, Dictyostelium discoideum. Differentiation 41:14-21. - PubMed
-
- Bonner, J. T., E. M. Hall, S. Noller, F. B. Oleson, Jr., and A. B. Roberts. 1972. Synthesis of cyclic AMP and phosphodiesterase in various species of cellular slime molds and its bearing on chemotaxis and differentiation. Dev. Biol. 29:402-409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

