Candida albicans lacking the gene encoding the regulatory subunit of protein kinase A displays a defect in hyphal formation and an altered localization of the catalytic subunit
- PMID: 14871949
- PMCID: PMC329502
- DOI: 10.1128/EC.3.1.190-199.2004
Candida albicans lacking the gene encoding the regulatory subunit of protein kinase A displays a defect in hyphal formation and an altered localization of the catalytic subunit
Abstract
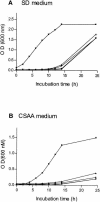
The fungal pathogen Candida albicans switches from a yeast-like to a filamentous mode of growth in response to a variety of environmental conditions. We examined the morphogenetic behavior of C. albicans yeast cells lacking the BCY1 gene, which encodes the regulatory subunit of protein kinase A. We cloned the BCY1 gene and generated a bcy1 tpk2 double mutant strain because a homozygous bcy1 mutant in a wild-type genetic background could not be obtained. In the bcy1 tpk2 mutant, protein kinase A activity (due to the presence of the TPK1 gene) was cyclic AMP independent, indicating that the cells harbored an unregulated phosphotransferase activity. This mutant has constitutive protein kinase A activity and displayed a defective germinative phenotype in N-acetylglucosamine and in serum-containing medium. The subcellular localization of a Tpk1-green fluorescent protein (GFP) fusion protein was examined in wild-type, tpk2 null, and bcy1 tpk2 double mutant strains. The fusion protein was observed to be predominantly nuclear in wild-type and tpk2 strains. This was not the case in the bcy1 tpk2 double mutant, where it appeared dispersed throughout the cell. Coimmunoprecipitation of Bcy1p with the Tpk1-GFP fusion protein demonstrated the interaction of these proteins inside the cell. These results suggest that one of the roles of Bcy1p is to tether the protein kinase A catalytic subunit to the nucleus.
Figures







References
-
- Bockmühl, D. P., S. Krishnamurthy, M. Gerards, A. Sonneborn, and J. F Ernst. 2001. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 42:1243-1257. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

