Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21
- PMID: 14872059
- PMCID: PMC373400
- DOI: 10.1093/nar/gkh265
Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21
Abstract
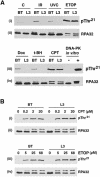
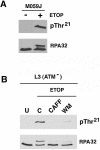
Replication protein A (RPA) is a single-stranded DNA (ssDNA) binding protein involved in various processes, including nucleotide excision repair and DNA replication. The 32 kDa subunit of RPA (RPA32) is phosphorylated in response to various DNA-damaging agents, and two protein kinases, ataxia-telangiectasia mutated (ATM) and the DNA-dependent protein kinase (DNA-PK) have been implicated in DNA damage-induced phosphorylation of RPA32. However, the relative roles of ATM and DNA-PK in the site-specific DNA damage-induced phosphorylation of RPA32 have not been reported. Here we generated a phosphospecific antibody that recognizes Thr21-phosphorylated RPA32. We show that both DNA-PK and ATM phosphorylate RPA32 on Thr21 in vitro. Ionizing radiation (IR)-induced phosphorylation of RPA32 on Thr21 was defective in ATM-deficient cells, while camptothecin (CPT)-induced phosphorylation of RPA32 on Thr21 was defective in cells lacking functional DNA-PK. Neither ATM nor DNA-PK was required for etoposide (ETOP)-induced RPA32 Thr21 phosphorylation. However, two inhibitors of the ATM- and Rad3-related (ATR) protein kinase activity prevented ETOP-induced Thr21 phosphorylation. Inhibition of DNA replication prevented both the IR- and CPT-induced phosphorylation of Thr21, whereas ETOP-induced Thr21 phosphorylation did not require active DNA replication. Thus, the regulation of RPA32 Thr21 phosphorylation by multiple DNA damage response protein kinases suggests that Thr21 phosphorylation of RPA32 is a crucial step within the DNA damage response.
Figures






References
-
- Wold M.S. (1997) Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem., 66, 61–92. - PubMed
-
- Din S., Brill,S.J., Fairman,M.P. and Stillman,B. (1990) Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev., 4, 968–977. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

