The genetics of geometry
- PMID: 14960734
- PMCID: PMC387316
- DOI: 10.1073/pnas.0306308101
The genetics of geometry
Abstract
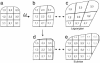
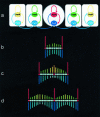
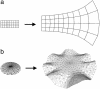
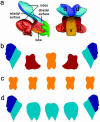
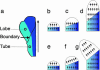
Although much progress has been made in understanding how gene expression patterns are established during development, much less is known about how these patterns are related to the growth of biological shapes. Here we describe conceptual and experimental approaches to bridging this gap, with particular reference to plant development where lack of cell movement simplifies matters. Growth and shape change in plants can be fully described with four types of regional parameter: growth rate, anisotropy, direction, and rotation. A key requirement is to understand how these parameters both influence and respond to the action of genes. This can be addressed by using mechanistic models that capture interactions among three components: regional identities, regionalizing morphogens, and polarizing morphogens. By incorporating these interactions within a growing framework, it is possible to generate shape changes and associated gene expression patterns according to particular hypotheses. The results can be compared with experimental observations of growth of normal and mutant forms, allowing further hypotheses and experiments to be formulated. We illustrate these principles with a study of snapdragon petal growth.
Figures
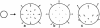
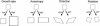




References
-
- Lawrence, P. A. (1992) The Making of a Fly (Blackwell Scientific, Oxford).
-
- Wolpert, L., Brockes, J., Jessell, T., Lawrence, P. & Meyerowitz, E. (1998) Principles of Development (Oxford Univ. Press, Oxford).
-
- Coen, E. (2000) The Art of Genes (Oxford Univ. Press, Oxford).
-
- Teleman, A., Strigini, M. & Cohen, S. M. (2001) Cell 105, 559–562. - PubMed
-
- Steeves, T. A. & Sussex, I. M. (1989) Patterns in Plant Development (Cambridge Univ. Press, Cambridge, U.K.).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

