MEPE has the properties of an osteoblastic phosphatonin and minhibin
- PMID: 14962809
- PMCID: PMC3357088
- DOI: 10.1016/j.bone.2003.10.005
MEPE has the properties of an osteoblastic phosphatonin and minhibin
Abstract
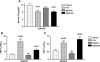
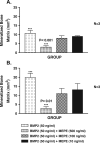
Matrix extracellular phosphoglycoprotein (MEPE) is expressed exclusively in osteoblasts, osteocytes and odontoblasts with markedly elevated expression found in X-linked hypophosphatemic rickets (Hyp) osteoblasts and in oncogenic hypophosphatemic osteomalacia (OHO) tumors. Because these syndromes are associated with abnormalities in mineralization and renal phosphate excretion, we examined the effects of insect-expressed full-length human-MEPE (Hu-MEPE) on serum and urinary phosphate in vivo, (33)PO(4) uptake in renal proximal tubule cultures and mineralization of osteoblast cultures. Dose-dependent hypophosphatemia and hyperphosphaturia occurred in mice following intraperitoneal (IP) administration of Hu-MEPE (up to 400 microg kg(-1) 31 h(-1)), similar to mice given the phosphaturic hormone PTH (80 microg kg(-1) 31 h(-1)). Also the fractional excretion of phosphate (FEP) was stimulated by MEPE [65.0% (P < 0.001)] and PTH groups [53.3% (P < 0.001)] relative to the vehicle group [28.7% (SEM 3.97)]. In addition, Hu-MEPE significantly inhibited (33)PO(4) uptake in primary human proximal tubule renal cells (RPTEC) and a human renal cell line (Hu-CL8) in vitro (V(max) 53.4% inhibition; K(m) 27.4 ng/ml, and V(max) 9.1% inhibition; K(m) 23.8 ng/ml, respectively). Moreover, Hu-MEPE dose dependently (50-800 ng/ml) inhibited BMP2-mediated mineralization of a murine osteoblast cell line (2T3) in vitro. Inhibition of mineralization was localized to a small (2 kDa) cathepsin B released carboxy-terminal MEPE peptide (protease-resistant) containing the acidic serine-aspartate-rich motif (ASARM peptide). We conclude that MEPE promotes renal phosphate excretion and modulates mineralization.
Figures




References
-
- Aisa MC, Rahman S, Senin U, Maggio D, Russell RG. Cathepsin B activity in normal human osteoblast-like cells and human osteoblastic osteosarcoma cells (MG-63): regulation by interleukin-1 beta and parathyroid hormone. Biochim Biophys Acta. 1996;1290:29–36. - PubMed
-
- Argiro L, Desbarats M, Glorieux FH, Ecarot B. Mepe, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics. 2001;74:342–51. - PubMed
-
- Bai X, Miao D, Panda D, Grady S, McKee MD, Goltzman D, et al. Partial rescue of the Hyp phenotype by osteoblast-targeted PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) expression. Mol Endocrinol. 2002;16:2913–25. - PubMed
-
- Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in FGF23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;7:7. - PubMed
-
- Bennick A, McLaughlin AC, Grey AA, Madapallimattam G. The location and nature of calcium-binding sites in salivary acidic pro-line-rich phosphoproteins. J Biol Chem. 1981;256:4741–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

