Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine
- PMID: 14963162
- PMCID: PMC369202
- DOI: 10.1128/jvi.78.5.2586-2590.2004
Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine
Abstract
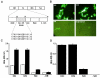
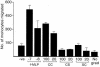
Human immunodeficiency virus type 1 (HIV-1)-associated dementia (HAD) is correlated with increased monocyte migration to the brain, and the incidence of HAD among otherwise asymptomatic subjects appears to be lower in India than in the United States and Europe (1 to 2% versus 15 to 30%). Because of the genetic differences between HIV-1 strains circulating in these regions, we sought to identify viral determinants associated with this difference. We targeted Tat protein for these studies in view of its association with monocyte chemotactic function. Analyses of Tat sequences representing nine subtypes revealed that at least six amino acid residues are differentially conserved in subtype C Tat (C-Tat). Of these, cysteine (at position 31) was highly (>99%) conserved in non-subtype C viruses and more than 90% of subtype C viruses encoded a serine. We hypothesized a compromised chemotactic function of C-Tat due to the disruption of CC motif and tested it with the wild type C-Tat (CS) and its two isogenic variants (CC and SC) derived by site-directed mutagenesis. We found that the CS natural variant was defective for monocyte chemotactic activity without a loss in the transactivation property. While the CC mutant is functionally competent for both the functions, in contrast, the SC mutant was defective in both. Therefore, the loss of the C-Tat chemotactic property may underlie the reduced incidence of HAD; although not presenting conclusive evidence, this study provides the first evidence for a potential epidemiologic phenomenon associated with biological differences in the subtype C viruses.
Figures
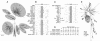
Similar articles
-
Human immunodeficiency virus type 1 subtype C exhibits higher transactivation activity of Tat than subtypes B and E.Microbiol Immunol. 2002;46(11):787-99. doi: 10.1111/j.1348-0421.2002.tb02766.x. Microbiol Immunol. 2002. PMID: 12516777
-
Transactivation and signaling functions of Tat are not correlated: biological and immunological characterization of HIV-1 subtype-C Tat protein.Retrovirology. 2006 Aug 18;3:53. doi: 10.1186/1742-4690-3-53. Retrovirology. 2006. PMID: 16916472 Free PMC article.
-
Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G.J Virol. 2000 Apr;74(8):3740-51. doi: 10.1128/jvi.74.8.3740-3751.2000. J Virol. 2000. PMID: 10729149 Free PMC article.
-
Multiple modes of transcriptional regulation by the HIV-1 Tat transactivator.IUBMB Life. 2001 Mar;51(3):175-81. doi: 10.1080/152165401753544241. IUBMB Life. 2001. PMID: 11547919 Review.
-
HIV-Tat dependent chemotaxis and invasion, key aspects of tat mediated pathogenesis.Clin Exp Metastasis. 2000;18(7):533-8. doi: 10.1023/a:1011991906685. Clin Exp Metastasis. 2000. PMID: 11688957 Review.
Cited by
-
Proceedings from the NIMH symposium on "NeuroAIDS in Africa: neurological and neuropsychiatric complications of HIV".J Neurovirol. 2016 Oct;22(5):699-702. doi: 10.1007/s13365-016-0467-y. Epub 2016 Jul 29. J Neurovirol. 2016. PMID: 27473196 Free PMC article.
-
Differential Contribution of HIV-1 Subtypes B and C to Neurological Disorders: Mechanisms and Possible Treatments.AIDS Rev. 2019;21(2):76-83. doi: 10.24875/AIDSRev.19000051. AIDS Rev. 2019. PMID: 31332398 Free PMC article. Review.
-
Human immunodeficiency virus type 1 subtype C Tat fails to induce intracellular calcium flux and induces reduced tumor necrosis factor production from monocytes.J Virol. 2007 Jun;81(11):5919-28. doi: 10.1128/JVI.01938-06. Epub 2007 Mar 21. J Virol. 2007. PMID: 17376903 Free PMC article.
-
The gp120 protein is a second determinant of decreased neurovirulence of Indian HIV-1C isolates compared to southern African HIV-1C isolates.PLoS One. 2014 Sep 4;9(9):e107074. doi: 10.1371/journal.pone.0107074. eCollection 2014. PLoS One. 2014. PMID: 25188269 Free PMC article.
-
Functional conservation and coherence of HIV-1 subtype A Vpu alleles.Sci Rep. 2017 Dec;7(1):87. doi: 10.1038/s41598-017-00222-8. Epub 2017 Mar 7. Sci Rep. 2017. PMID: 28273896 Free PMC article.
References
-
- Albini, A., R. Benelli, D. Giunciuglio, T. Cai, G. Mariani, S. Ferrini, and D. M. Noonan. 1998. Identification of a novel domain of HIV tat involved in monocyte chemotaxis. J. Biol. Chem. 273:15895-15900. - PubMed
-
- Benelli, R., A. Barbero, S. Ferrini, P. Scapini, M. Cassatella, F. Bussolino, C. Tacchetti, D. M. Noonan, and A. Albini. 2000. Human immunodeficiency virus transactivator protein (Tat) stimulates chemotaxis, calcium mobilization, and activation of human polymorphonuclear leukocytes: implications for Tat-mediated pathogenesis. J. Infect. Dis. 182:1643-1651. - PubMed
-
- Benelli, R., R. Mortarini, A. Anichini, D. Giunciuglio, D. M. Noonan, S. Montalti, C. Tacchetti, and A. Albini. 1998. Monocyte-derived dendritic cells and monocytes migrate to HIV-Tat RGD and basic peptides. AIDS 12:261-268. - PubMed
-
- Bonwetsch, R., S. Croul, M. W. Richardson, C. Lorenzana, L. D. Valle, A. E. Sverstiuk, S. Amini, S. Morgello, K. Khalili, and J. Rappaport. 1999. Role of HIV-1 Tat and CC chemokine MIP-1α in the pathogenesis of HIV associated central nervous system disorders. J. Neurovirol. 5:685-694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

