Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis
- PMID: 14963232
- PMCID: PMC365766
- DOI: 10.1073/pnas.0308600100
Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis
Abstract
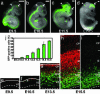
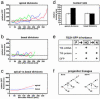
Neurons of the mammalian CNS are thought to originate from progenitors dividing at the apical surface of the neuroepithelium. Here we use mouse embryos expressing GFP from the Tis21 locus, a gene expressed throughout the neural tube in most, if not all, neuron-generating progenitors, to specifically reveal the cell divisions that produce CNS neurons. In addition to the apical, asymmetric divisions of neuroepithelial (NE) cells that generate another NE cell and a neuron, we find, from the onset of neurogenesis, a second population of progenitors that divide in the basal region of the neuroepithelium and generate two neurons. Basal progenitors are most frequent in the telencephalon, where they outnumber the apically dividing neuron-generating NE cells. Our observations reconcile previous data on the origin and lineage of CNS neurons and show that basal, rather than apical, progenitors are the major source of the neurons of the mammalian neocortex.
Figures


References
-
- Rakic, P. (1988) Science 241, 170-176. - PubMed
-
- McConnell, S. K. (1995) Neuron 15, 761-768. - PubMed
-
- Huttner, W. B. & Brand, M. (1997) Curr. Opin. Neurobiol. 7, 29-39. - PubMed
-
- Caviness, V. S., Takahashi, T. & Nowakowski, R. S. (2000) in Results and Problems in Cell Differentiation, Mouse Brain Development, eds. Goffinet, A. M. & Rakic, P. (Springer, Berlin), Vol. 30, pp. 107-143. - PubMed
-
- Cai, L., Hayes, N. L., Takahashi, T., Caviness, V. S., Jr., & Nowakowski, R. S. (2002) J. Neurosci. Res. 69, 731-744. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

