NopL, an effector protein of Rhizobium sp. NGR234, thwarts activation of plant defense reactions
- PMID: 14966249
- PMCID: PMC344561
- DOI: 10.1104/pp.103.031740
NopL, an effector protein of Rhizobium sp. NGR234, thwarts activation of plant defense reactions
Abstract
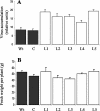
Bacterial effector proteins delivered into eukaryotic cells via bacterial type III secretion systems are important virulence factors in plant-pathogen interactions. Type III secretion systems have been found in Rhizobium species that form symbiotic, nitrogen-fixing associations with legumes. One such bacterium, Rhizobium sp. NGR234, secretes a number of type III effectors, including nodulation outer protein L (NopL, formerly y4xL). Here, we show that expression of nopL in tobacco (Nicotiana tabacum) prevents full induction of pathogenesis-related (PR) defense proteins. Transgenic tobacco plants that express nopL and were infected with potato virus Y (necrotic strain 605) exhibited only very low levels of chitinase (class I) and beta-1,3-glucanase (classes I and III) proteins. Northern-blot analysis indicated that expression of nopL in plant cells suppresses transcription of PR genes. Treatment with ethylene counteracted the effect of NopL on chitinase (class I). Transgenic Lotus japonicus plants that expressed nopL exhibited delayed development and low chitinase levels. In vitro experiments showed that NopL is a substrate for plant protein kinases. Together, these data suggest that NopL, when delivered into the plant cell, modulates the activity of signal transduction pathways that culminate in activation of PR proteins.
Figures





References
-
- Ausubel FM, Brent R, Kingston RE, More DD, Seidman JG, Smith JA, Struhl K (1990) Current Protocols in Molecular Biology, Vol. 1 (Supplement 9). John Wiley & Sons, New York
-
- Axtell MJ, Staskawicz BJ (2003) Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112: 369–377 - PubMed
-
- Bartsev AV, Boukli NM, Deakin WJ, Staehelin C, Broughton WJ (2003) Purification and phosphorylation of the effector protein NopL from Rhizobium sp. NGR234. FEBS Lett 554: 271–274 - PubMed
-
- Boller T, Gehri A, Mauch F, Vögeli U (1983) Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta 157: 22–31 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

