The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction
- PMID: 14966267
- PMCID: PMC350558
- DOI: 10.1128/MCB.24.5.1844-1854.2004
The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction
Abstract
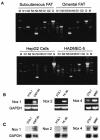
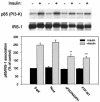
Insulin stimulation of target cells elicits a burst of H(2)O(2) that enhances tyrosine phosphorylation of the insulin receptor and its cellular substrate proteins as well as distal signaling events in the insulin action cascade. The molecular mechanism coupling the insulin receptor with the cellular oxidant-generating apparatus has not been elucidated. Using reverse transcription-PCR and Northern blot analyses, we found that Nox4, a homolog of gp91phox, the phagocytic NAD(P)H oxidase catalytic subunit, is prominently expressed in insulin-sensitive adipose cells. Adenovirus-mediated expression of Nox4 deletion constructs lacking NAD(P)H or FAD/NAD(P)H cofactor binding domains acted in a dominant-negative fashion in differentiated 3T3-L1 adipocytes and attenuated insulin-stimulated H(2)O(2) generation, insulin receptor (IR) and IRS-1 tyrosine phosphorylation, activation of downstream serine kinases, and glucose uptake. Transfection of specific small interfering RNA oligonucleotides reduced Nox4 protein abundance and also inhibited the insulin signaling cascade. Overexpression of Nox4 also significantly reversed the inhibition of insulin-stimulated IR tyrosine phosphorylation induced by coexpression of PTP1B by inhibiting PTP1B catalytic activity. These data suggest that Nox4 provides a novel link between the IR and the generation of cellular reactive oxygen species that enhance insulin signal transduction, at least in part via the oxidative inhibition of cellular protein-tyrosine phosphatases (PTPases), including PTP1B, a PTPase that has been previously implicated in the regulation of insulin action.
Figures








References
-
- Babior, B. M., J. D. Lambeth, and W. Nauseef. 2002. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397:342-344. - PubMed
-
- Bae, Y. S., S. W. Kang, M. S. Seo, I. C. Baines, E. Tekle, P. B. Chock, and S. G. Rhee. 1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272:217-221. - PubMed
-
- Bae, Y. S., J. Y. Sung, O. S. Kim, Y. J. Kim, K. C. Hur, A. Kazlauskas, and S. G. Rhee. 2000. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275:10527-10531. - PubMed
-
- Banfi, B., R. A. Clark, K. Steger, and K. H. Krause. 2003. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 278:3510-3513. - PubMed
-
- Barrett, W. C., J. P. DeGnore, Y. F. Keng, Z. Y. Zhang, M. B. Yim, and P. B. Chock. 1999. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J. Biol. Chem. 274:34543-34546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
