Replication protein A (RPA) phosphorylation prevents RPA association with replication centers
- PMID: 14966274
- PMCID: PMC350552
- DOI: 10.1128/MCB.24.5.1930-1943.2004
Replication protein A (RPA) phosphorylation prevents RPA association with replication centers
Abstract
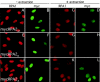
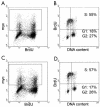
Mammalian replication protein A (RPA) undergoes DNA damage-dependent phosphorylation at numerous sites on the N terminus of the RPA2 subunit. To understand the functional significance of RPA phosphorylation, we expressed RPA2 variants in which the phosphorylation sites were converted to aspartate (RPA2(D)) or alanine (RPA2(A)). Although RPA2(D) was incorporated into RPA heterotrimers and supported simian virus 40 DNA replication in vitro, the RPA2(D) mutant was selectively unable to associate with replication centers in vivo. In cells containing greatly reduced levels of endogenous RPA2, RPA2(D) again did not localize to replication sites, indicating that the defect in supporting chromosomal DNA replication is not due to competition with the wild-type protein. Use of phosphospecific antibodies demonstrated that endogenous hyperphosphorylated RPA behaves similarly to RPA2(D). In contrast, under DNA damage or replication stress conditions, RPA2(D), like RPA2(A) and wild-type RPA2, was competent to associate with DNA damage foci as determined by colocalization with gamma-H2AX. We conclude that RPA2 phosphorylation prevents RPA association with replication centers in vivo and potentially serves as a marker for sites of DNA damage.
Figures







References
-
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
-
- Bartek, J., and J. Lukas. 2001. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 13:738-747. - PubMed
-
- Binz, S. K., Y. Lao, D. F. Lowry, and M. S. Wold. 2003. The phosphorylation domain of the 32-kDa subunit of replication protein A modulates RPA-DNA interactions: evidence for an intersubunit interaction. J. Biol. Chem. 278:35584-35591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
