Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion
- PMID: 14966279
- PMCID: PMC350540
- DOI: 10.1128/MCB.24.5.1990-1999.2004
Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion
Abstract
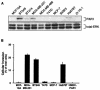
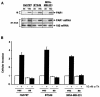
Increased expression of protease-activated receptor 1 (PAR1), a G protein-coupled receptor for thrombin, has previously been correlated with breast carcinoma cell invasion. PAR1 is irreversibly proteolytically activated, internalized, and sorted directly to lysosomes, a critical process for the termination of signaling. We determined that activated PAR1 trafficking is severely altered in metastatic breast carcinoma cells but not in nonmetastatic or normal breast epithelial cells. Consequently, the proteolytically activated receptor is not sorted to lysosomes and degraded. Altered trafficking of proteolytically activated PAR1 caused sustained activation of phosphoinositide hydrolysis and extracellular signal-regulated kinase signaling, even after thrombin withdrawal, and enhanced cellular invasion. Thus, our results reveal that a novel alteration in trafficking of activated PAR1 causes persistent signaling and, in addition to other processes and proteins, contributes to breast carcinoma cell invasion.
Figures
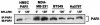



References
-
- Albini, A., Y. Iwamoto, H. K. Kleinman, G. R. Martin, S. A. Aaronson, J. M. Kozlowski, and R. N. McEwan. 1987. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 47:3239-3245. - PubMed
-
- Bar-Sagi, D., and J. R. Feramisco. 1986. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233:1061-1068. - PubMed
-
- Christoforidis, S., H. M. McBride, R. D. Burgoyne, and M. Zerial. 1999. The Rab5 effector EEA1 is a core component of endosome docking. Nature 397:621-625. - PubMed
-
- Clark, G. J., and C. J. Der. 1995. Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res. Treat. 35:133-144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
