Activation of GABA(A) receptors containing the alpha4 subunit by GABA and pentobarbital
- PMID: 14966300
- PMCID: PMC1664939
- DOI: 10.1113/jphysiol.2003.058230
Activation of GABA(A) receptors containing the alpha4 subunit by GABA and pentobarbital
Abstract
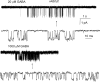
The activation properties of GABA(A) receptors containing alpha4beta2gamma2 and alpha4beta2delta subunits were examined in the presence of GABA or pentobarbital. The receptors were expressed transiently in HEK 293 cells, and the electrophysiological experiments were carried out using cell-attached single-channel patch clamp or whole-cell macroscopic recordings. The data show that GABA is a stronger activator of alpha4beta2gamma2 receptors than alpha4beta2delta receptors. Single-channel clusters were recorded from alpha4beta2gamma2 receptors in the presence of 10-5000 microm GABA. The maximal intracluster open probability was 0.35, with a half-maximal response elicited by 32 microm GABA. Simultaneous kinetic analysis of single-channel currents obtained at various GABA concentrations yields a channel opening rate constant of 250 s(-1), and a K(D) of 20 microm. In contrast, only isolated openings were observed in the presence of GABA for the alpha4beta2delta receptor. Pentobarbital was a strong activator of both alpha4beta2gamma2 and alpha4beta2delta receptors. The maximal cluster open probability, recorded in the presence of 100 microm pentobarbital, was 0.7. At higher pentobarbital concentrations, the cluster open probability was reduced, probably due to channel block. The results from single-channel experiments were confirmed by macroscopic recordings from HEK cells in the presence of GABA or pentobarbital.
Figures

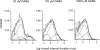



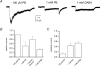
Similar articles
-
Modulation of GABA(A) receptor channel gating by pentobarbital.J Physiol. 2001 Dec 15;537(Pt 3):715-33. doi: 10.1111/j.1469-7793.2001.00715.x. J Physiol. 2001. PMID: 11744750 Free PMC article.
-
Pregnenolone sulfate block of GABA(A) receptors: mechanism and involvement of a residue in the M2 region of the alpha subunit.J Physiol. 2001 May 1;532(Pt 3):673-84. doi: 10.1111/j.1469-7793.2001.0673e.x. J Physiol. 2001. PMID: 11313438 Free PMC article.
-
Structural domains of the human GABAA receptor 3 subunit involved in the actions of pentobarbital.J Physiol. 2000 May 1;524 Pt 3(Pt 3):649-76. doi: 10.1111/j.1469-7793.2000.00649.x. J Physiol. 2000. PMID: 10790149 Free PMC article.
-
Kinetic properties of alpha 1 beta 1 gamma-aminobutyric acidA receptor channels expressed in Chinese hamster ovary cells: regulation by pentobarbital and picrotoxin.Mol Pharmacol. 1992 Nov;42(5):872-81. Mol Pharmacol. 1992. PMID: 1331767
-
Molecular basis for modulation of recombinant alpha1beta2gamma2 GABAA receptors by protons.J Neurophysiol. 2004 Aug;92(2):883-94. doi: 10.1152/jn.01040.2003. Epub 2004 Mar 17. J Neurophysiol. 2004. PMID: 15028749
Cited by
-
Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta GABAA receptors.J Neurosci. 2006 Feb 1;26(5):1499-506. doi: 10.1523/JNEUROSCI.2913-05.2006. J Neurosci. 2006. PMID: 16452673 Free PMC article.
-
Chemogenetic Isolation Reveals Synaptic Contribution of δ GABAA Receptors in Mouse Dentate Granule Neurons.J Neurosci. 2018 Sep 19;38(38):8128-8145. doi: 10.1523/JNEUROSCI.0799-18.2018. Epub 2018 Aug 3. J Neurosci. 2018. PMID: 30076210 Free PMC article.
-
Contrasting actions of a convulsant barbiturate and its anticonvulsant enantiomer on the α1 β3 γ2L GABAA receptor account for their in vivo effects.J Physiol. 2015 Nov 15;593(22):4943-61. doi: 10.1113/JP270971. J Physiol. 2015. PMID: 26378885 Free PMC article.
-
Ethanol effects on GABA-gated current in a model of increased alpha4betadelta GABAA receptor expression depend on time course and preexposure to low concentrations of the drug.Alcohol. 2007 May;41(3):223-31. doi: 10.1016/j.alcohol.2007.04.007. Alcohol. 2007. PMID: 17591545 Free PMC article.
-
Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors.J Physiol. 2010 Apr 15;588(Pt 8):1251-68. doi: 10.1113/jphysiol.2009.182444. Epub 2010 Feb 22. J Physiol. 2010. PMID: 20176630 Free PMC article.
References
-
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. - PubMed
-
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β ssubunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

