Learning-induced improvement in encoding and decoding of specific movement directions by neurons in the primary motor cortex
- PMID: 14966539
- PMCID: PMC340948
- DOI: 10.1371/journal.pbio.0020045
Learning-induced improvement in encoding and decoding of specific movement directions by neurons in the primary motor cortex
Abstract
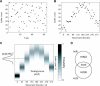
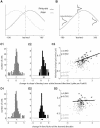
Many recent studies describe learning-related changes in sensory and motor areas, but few have directly probed for improvement in neuronal coding after learning. We used information theory to analyze single-cell activity from the primary motor cortex of monkeys, before and after learning a local rotational visuomotor task. We show that after learning, neurons in the primary motor cortex conveyed more information about the direction of movement and did so with relation to their directional sensitivity. Similar to recent findings in sensory systems, this specific improvement in encoding is correlated with an increase in the slope of the neurons' tuning curve. We further demonstrate that the improved information after learning enables a more accurate reconstruction of movement direction from neuronal populations. Our results suggest that similar mechanisms govern learning in sensory and motor areas and provide further evidence for a tight relationship between the locality of learning and the properties of neurons; namely, cells only show plasticity if their preferred direction is near the training one. The results also suggest that simple learning tasks can enhance the performance of brain-machine interfaces.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures


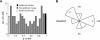

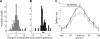



References
-
- Alexander GE, Crutcher MD. Preparation for movement: Neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. - PubMed
-
- Buracas GT, Zador AM, DeWeese MR, Albright TD. Efficient discrimination of temporal patterns by motion-sensitive neurons in primate visual cortex. Neuron. 1998;20:959–969. - PubMed
-
- Chen LL, Wise SP. Conditional oculomotor learning: Population vectors in the supplementary eye field. J Neurophysiol. 1997;78:1166–1169. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

