Inactivation of Icmt inhibits transformation by oncogenic K-Ras and B-Raf
- PMID: 14966563
- PMCID: PMC338259
- DOI: 10.1172/JCI18829
Inactivation of Icmt inhibits transformation by oncogenic K-Ras and B-Raf
Abstract
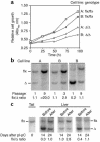
Isoprenylcysteine carboxyl methyltransferase (Icmt) methylates the carboxyl-terminal isoprenylcysteine of CAAX proteins (e.g., Ras and Rho proteins). In the case of the Ras proteins, carboxyl methylation is important for targeting of the proteins to the plasma membrane. We hypothesized that a knockout of Icmt would reduce the ability of cells to be transformed by K-Ras. Fibroblasts harboring a floxed Icmt allele and expressing activated K-Ras (K-Ras-Icmt(flx/flx)) were treated with Cre-adenovirus, producing K-Ras-Icmt(Delta/Delta) fibroblasts. Inactivation of Icmt inhibited cell growth and K-Ras-induced oncogenic transformation, both in soft agar assays and in a nude mice model. The inactivation of Icmt did not affect growth factor-stimulated phosphorylation of Erk1/2 or Akt1. However, levels of RhoA were greatly reduced as a consequence of accelerated protein turnover. In addition, there was a large Ras/Erk1/2-dependent increase in p21(Cip1), which was probably a consequence of the reduced levels of RhoA. Deletion of p21(Cip1) restored the ability of K-Ras-Icmt(Delta/Delta) fibroblasts to grow in soft agar. The effect of inactivating Icmt was not limited to the inhibition of K-Ras-induced transformation: inactivation of Icmt blocked transformation by an oncogenic form of B-Raf (V599E). These studies identify Icmt as a potential target for reducing the growth of K-Ras- and B-Raf-induced malignancies.
Figures







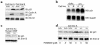


Comment in
-
Fighting cancer by disrupting C-terminal methylation of signaling proteins.J Clin Invest. 2004 Feb;113(4):513-5. doi: 10.1172/JCI21059. J Clin Invest. 2004. PMID: 14966560 Free PMC article.
References
-
- Casey PJ, Seabra MC. Protein prenyltransferases. J. Biol. Chem. 1996;271:5289–5292. - PubMed
-
- Boyartchuk VL, Ashby MN, Rine J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997;275:1796–1800. - PubMed
-
- Dai Q, et al. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J. Biol. Chem. 1998;273:15030–15034. - PubMed
-
- Reiss Y, Goldstein JL, Seabra MC, Casey PJ, Brown MS. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990;62:81–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

