Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors
- PMID: 14966573
- PMCID: PMC338268
- DOI: 10.1172/JCI20518
Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors
Abstract
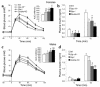
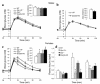
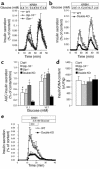
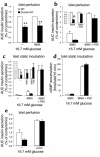
The role of the gluco-incretin hormones GIP and GLP-1 in the control of beta cell function was studied by analyzing mice with inactivation of each of these hormone receptor genes, or both. Our results demonstrate that glucose intolerance was additively increased during oral glucose absorption when both receptors were inactivated. After intraperitoneal injections, glucose intolerance was more severe in double- as compared to single-receptor KO mice, and euglycemic clamps revealed normal insulin sensitivity, suggesting a defect in insulin secretion. When assessed in vivo or in perfused pancreas, insulin secretion showed a lack of first phase in Glp-1R(-/-) but not in Gipr(-/-) mice. In perifusion experiments, however, first-phase insulin secretion was present in both types of islets. In double-KO islets, kinetics of insulin secretion was normal, but its amplitude was reduced by about 50% because of a defect distal to plasma membrane depolarization. Thus, gluco-incretin hormones control insulin secretion (a) by an acute insulinotropic effect on beta cells after oral glucose absorption (b) through the regulation, by GLP-1, of in vivo first-phase insulin secretion, probably by an action on extra-islet glucose sensors, and (c) by preserving the function of the secretory pathway, as evidenced by a beta cell autonomous secretion defect when both receptors are inactivated.
Figures



References
-
- Ebert R, Creutzfeldt W. Gastrointestinal peptides and insulin secretion. Diabetes Metab. Rev. 1987;3:1–16. - PubMed
-
- Creutzfeldt W, Nauck M. Gut hormones and diabetes mellitus. Diabetes Metab. Rev. 1992;8:149–177. - PubMed
-
- Weir GC, Mojsov S, Hendrick GK, Habener JF. Glucagon-like peptide 1 (7–37) actions on endocrine pancreas. Diabetes. 1989;38:338–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

