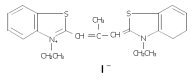
Inhibition of DNA primase and induction of apoptosis by 3,3'-diethyl-9-methylthia-carbocyanine iodide in hepatocellular carcinoma BEL-7402 cells
- PMID: 14966908
- PMCID: PMC4716971
- DOI: 10.3748/wjg.v10.i4.514
Inhibition of DNA primase and induction of apoptosis by 3,3'-diethyl-9-methylthia-carbocyanine iodide in hepatocellular carcinoma BEL-7402 cells
Abstract
Aim: To evaluate the effects of 3,3'-diethyl-9-methylthia-carbocyanine iodide (DMTCCI) on DNA primase activity and on apoptosis of human hepatocellular carcinoma BEL-7402 cells.
Methods: DNA primase assay was used to investigate DNA primase activity. MTT assay was applied to determine cell proliferation. Flow cytometric analysis, transmission electron microscopy, DNA fragmentation assay were performed to detect DMTCCI-induced apoptosis. Expression levels of p53, Bcl-2, Bcl-xL, Bad, Bax, survivin, Caspase-3 and poly (ADP-ribose) polymerase (PARP) were evaluated by immunoblot analysis. Caspase-3 activity was assessed with ApoAlert Caspase-3 colorimetric assay kit.
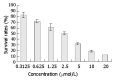
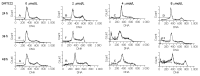
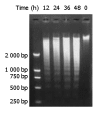
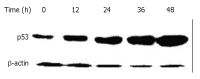
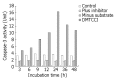
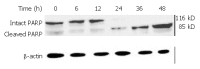
Results: DMTCCI had inhibitory effects on eukaryotic DNA primase activity with IC(50) value of 162.2 nmol/L. It also inhibited proliferation of human hepatocellular carcinoma BEL-7402 cells with IC(50) value of 2.09 micromol/L. Furthermore, DMTCCI-induced BEL-7402 cell apoptosis was confirmed by DNA fragmentation (DNA ladders and sub-G1 formation) and transmission electron microscopy (apoptotic bodies formation). During the induction of apoptosis, expression of Bcl-2, Bcl-xL and survivin was decreased, and that of p53, Bad and Bax was increased. Caspase-3 was activated and poly (ADP-ribose) polymerase (PARP) was cleaved in BEL-7402 cells treated with DMTCCI.
Conclusion: The present data suggest that DMTCCI has inhibitory effects on eukaryotic DNA primase and can induce apoptosis of BEL-7402 cells. The modulation of expression of p53 and Bcl-2 family proteins, and activation of Caspase-3 might be involved in the induction of apoptosis.
Figures






References
-
- Arezi B, Kuchta RD. Eukaryotic DNA primase. Trends Biochem Sci. 2000;25:572–576. - PubMed
-
- Cloutier S, Hamel H, Champagne M, Yotov WV. Mapping of the human DNA primase 1 (PRIM1) to chromosome 12q13. Genomics. 1997;43:398–401. - PubMed
-
- Grosse F, Krauss G. The primase activity of DNA polymerase alpha from calf thymus. J Biol Chem. 1985;260:1881–1888. - PubMed
-
- Simbulan CM, Tamiya-Koizumi K, Suzuki M, Shoji M, Taki T, Yoshida S. Sphingosine inhibits the synthesis of RNA primers by primase in vitro. Biochemistry. 1994;33:9007–9012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

