Copper binding in the prion protein
- PMID: 14967054
- PMCID: PMC2907897
- DOI: 10.1021/ar0301678
Copper binding in the prion protein
Abstract
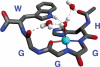
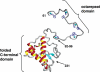
A conformational change of the prion protein is responsible for a class of neurodegenerative diseases called the transmissible spongiform encephalopathies that include mad cow disease and the human afflictions kuru and Creutzfeldt-Jakob disease. Despite the attention given to these diseases, the normal function of the prion protein in healthy tissue is unknown. Research over the past few years, however, demonstrates that the prion protein is a copper binding protein with high selectivity for Cu(2+). The structural features of the Cu(2+) binding sites have now been characterized and are providing important clues about the normal function of the prion protein and perhaps how metals or loss of protein function play a role in disease. The link between prion protein and copper may provide insight into the general, and recently appreciated, role of metals in neurodegenerative disease.
Figures

References
-
- Prusiner SB. Prion diseases and the BSE crisis. Science. 1997;278:245–251. - PubMed
-
- Dobson CM. Protein misfolding, evolution and disease. Trends Biochem. Sci. 1999;24:329–332. - PubMed
-
- Caughey B, Chesebro B. Prion Protein and the Transmissible Spongiform Encephalopathies. Trends Cell Biol. 1997;7:56–62. - PubMed
-
- Sailer A, Bueler H, Fischer M, Aguzzi A, Weissmann C. No propagation of prions in mice devoid of PrP. Cell. 1994;77:967–968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

